This document contains a 45 question chemistry exam from 1994 with multiple choice answers for each question. The questions cover a wide range of chemistry topics including organic chemistry reactions, properties of acids and bases, transition metals, thermodynamics, kinetics, and other general chemistry concepts.





















![29 Problem
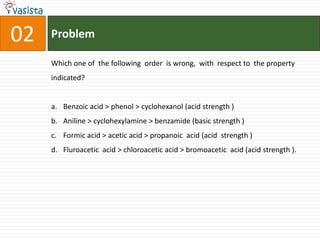
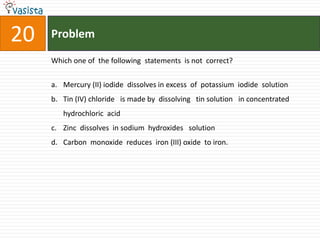
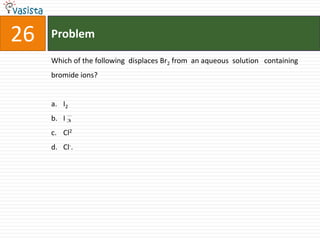
Which of the following salt has the same value of vant’s Hoff factor I
as that of K3[Fe(CN)6]?
a. Na2SO4
b. Al(NO3)3
c. Al2(SO4)3
d. NaCl](https://image.slidesharecdn.com/chemistry1994-111012012625-phpapp02/85/AIPMTChemistry-1994-31-320.jpg)
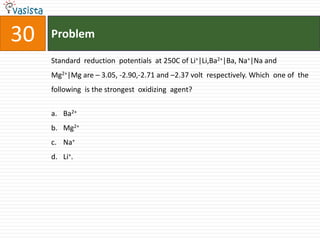
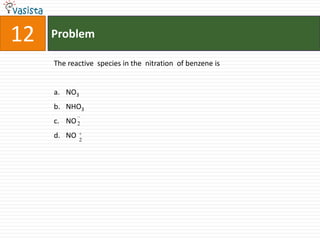
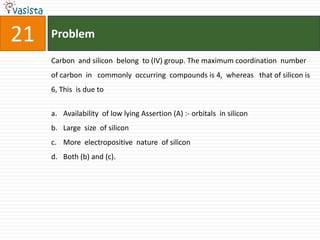
![31 Problem
The data for the reaction A + B → C, is
Exp. [A]0 [B]0 Initial rate
1 0.012 0.035 0.10
2 0.024 0.070 0.80
3 0.024 0.035 0.10
4 0.012 0.070 0.80
the rate law corresponds to the above data is
a. rate = k[A][B]3
b. rate = k [A]2[B]2
c. rate = k[B]3
d. rate = k[B]4](https://image.slidesharecdn.com/chemistry1994-111012012625-phpapp02/85/AIPMTChemistry-1994-33-320.jpg)
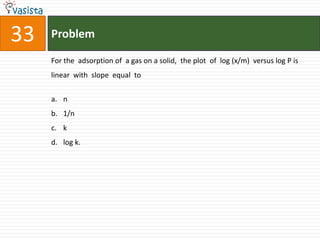

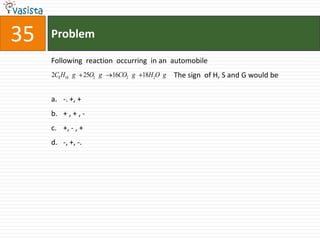










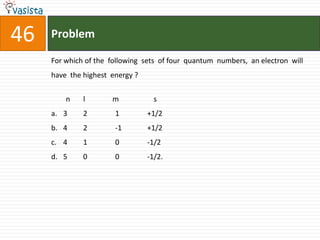

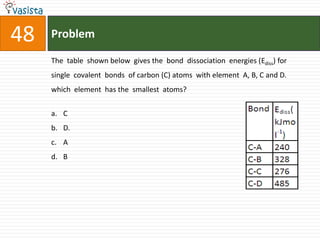
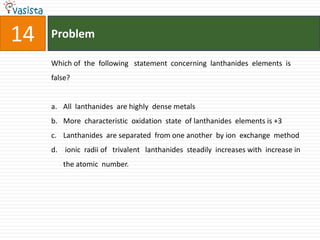
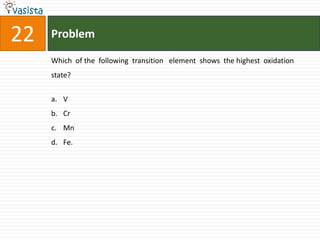
![49 Problem
At 800C, distilled water has [H3O+] concentration equal to 1 x 10-6 mole/litre.
O P F 1, The value of Kw at this temperature will be
a. 1 x 10-12
b. 1 x 10-15
c. 1 x 10-6
d. 1 x 10-9](https://image.slidesharecdn.com/chemistry1994-111012012625-phpapp02/85/AIPMTChemistry-1994-51-320.jpg)

