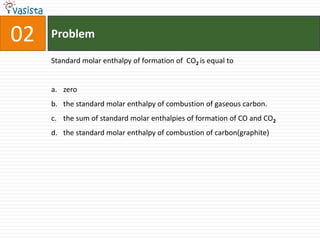
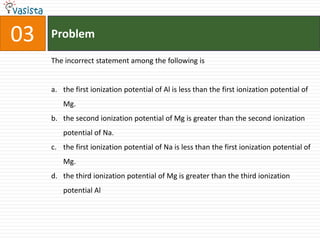
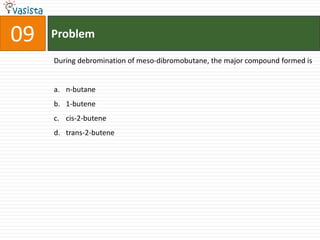
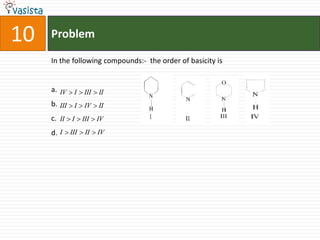
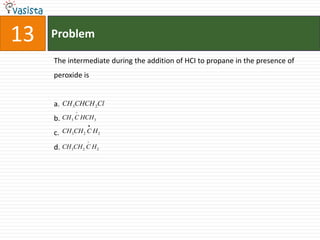
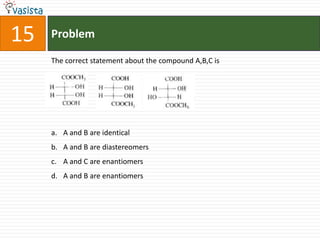
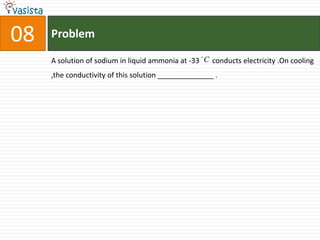
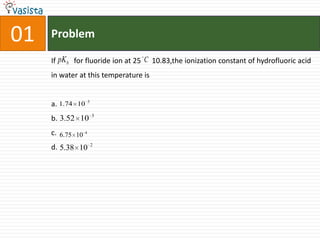
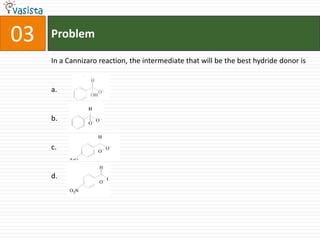
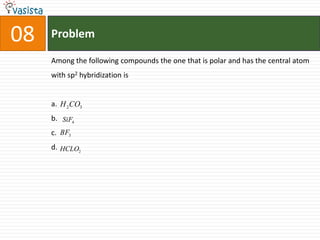
This document contains an unsolved chemistry paper from 1997 containing multiple choice questions testing knowledge of chemistry concepts. The questions cover topics such as molar heat capacity, standard enthalpies of formation, ionization potentials, properties that increase with atomic number for alkaline earth metals, mechanisms of nitration reactions, stereochemistry, solubility products, and identification of ions. There are three sections with a total of 30 questions testing understanding of fundamental chemistry principles.