The document contains 50 multiple choice questions from an unsolved chemistry past paper from 1999. The questions cover a range of chemistry topics including organic chemistry reactions, properties of elements, acid-base chemistry, stoichiometry, gas laws, and more. The full solutions to the questions are not provided in the document.



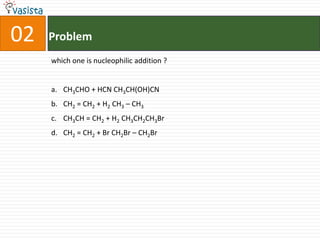

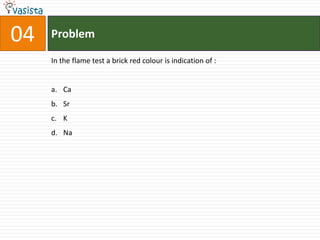
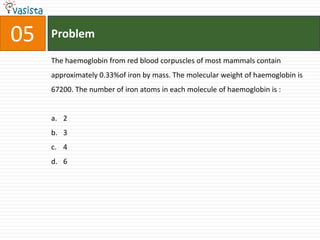
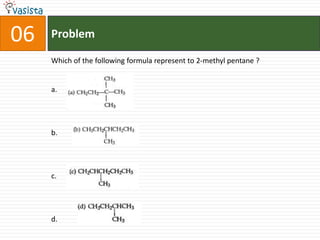
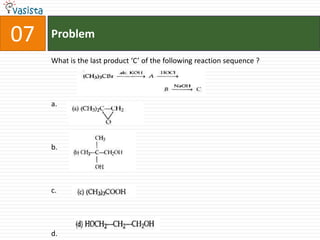











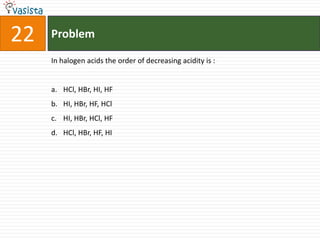


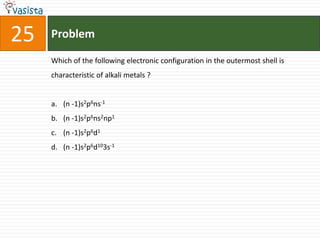





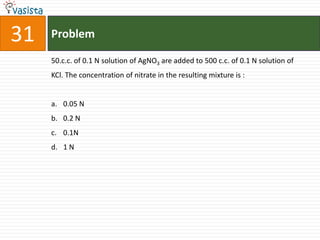


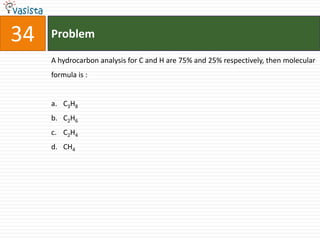


![37 Problem
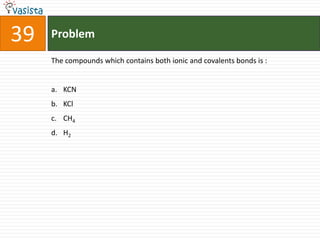
Oxidising number of Fe in K3[Fe(CN)6]
a. + 1
b. + 2
c. + 3
d. + 4](https://image.slidesharecdn.com/chemistry-1999-120103060921-phpapp01/85/AMU-Chemistry-1999-39-320.jpg)