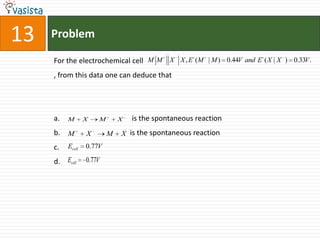
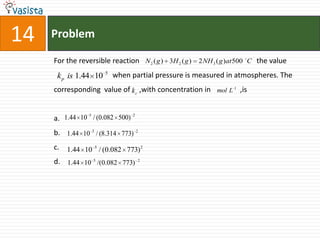
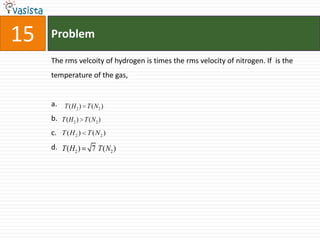
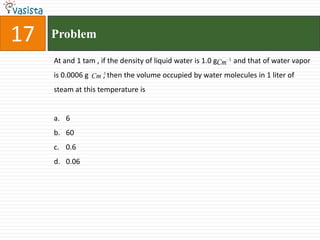
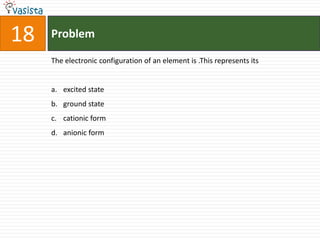
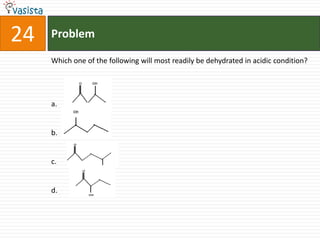
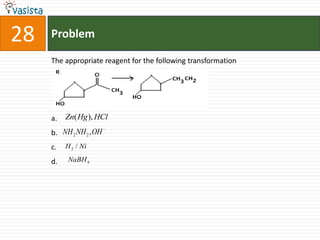
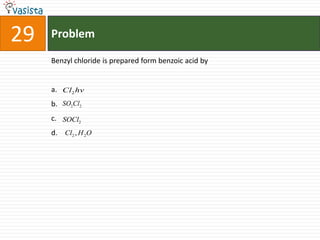
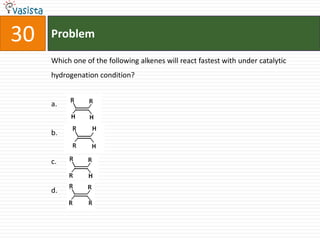
This document contains a past paper from the 2000 IIT JEE chemistry exam. It has two sections - Section I contains 25 multiple choice questions about various chemistry concepts. Section II contains 5 reasoning type questions where students must evaluate if an assertion and corresponding reason are both correct or incorrect. The document provides questions and problems about topics like oxidation states, acid strength, molecular geometry, hybridization, reaction mechanisms, isomerism, and properties of gases.