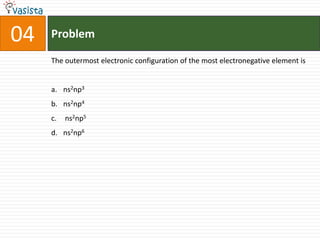
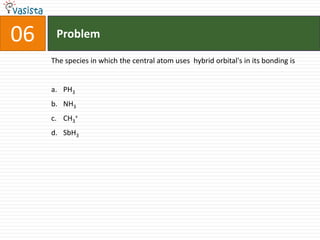
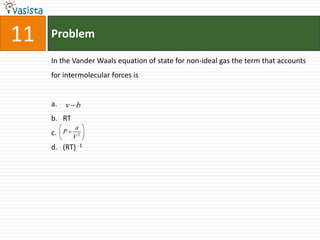
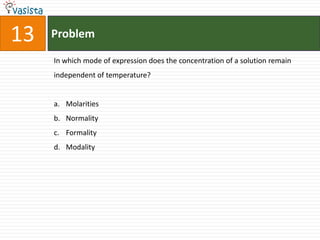
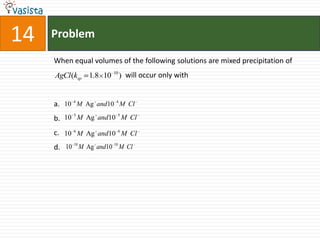
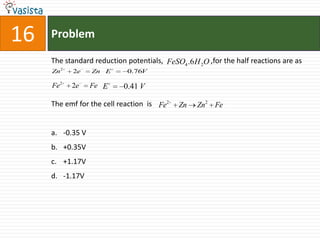
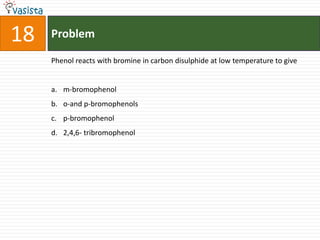
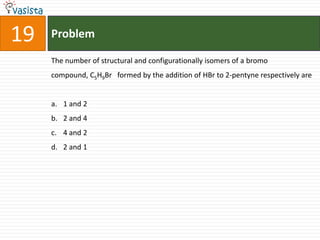
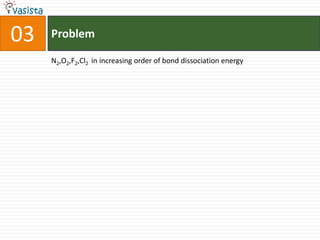
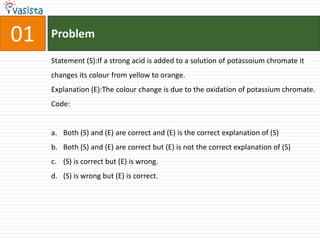
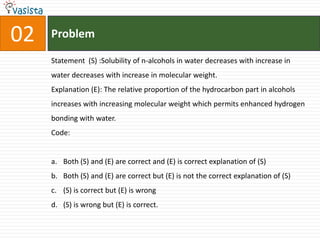
1) The document contains an unsolved chemistry exam from 1988 consisting of multiple choice, fill-in-the-blank, matching, and ordering questions testing knowledge of chemistry concepts and principles.
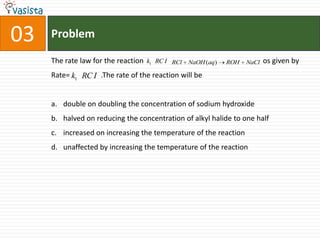
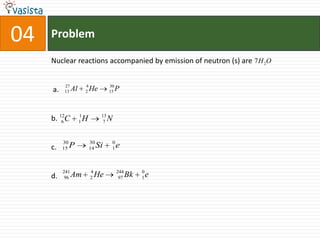
2) The multiple choice sections cover topics like electronic configurations, ionization potentials, molecular structures, and acid-base chemistry.
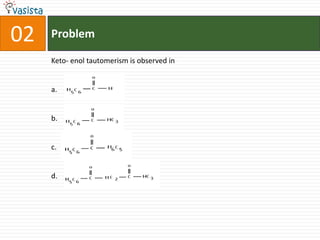
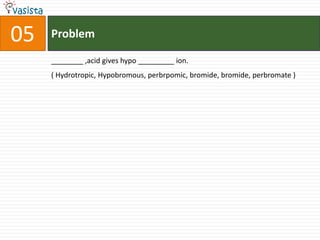
3) Fill-in-the-blank questions require completing statements about quantum mechanics concepts, extractive metallurgy processes, and organic compound classifications.
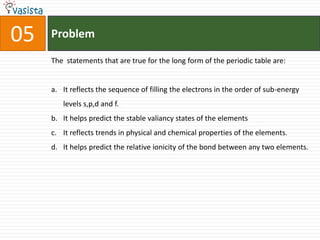
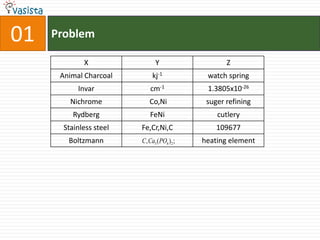
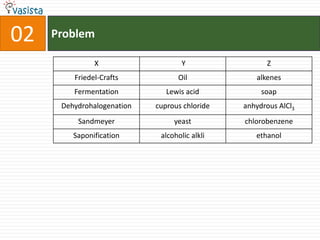
4) Other sections include matching elements to properties, ordering substances by thermal stability or other trends, and identifying correct statements and explanations.