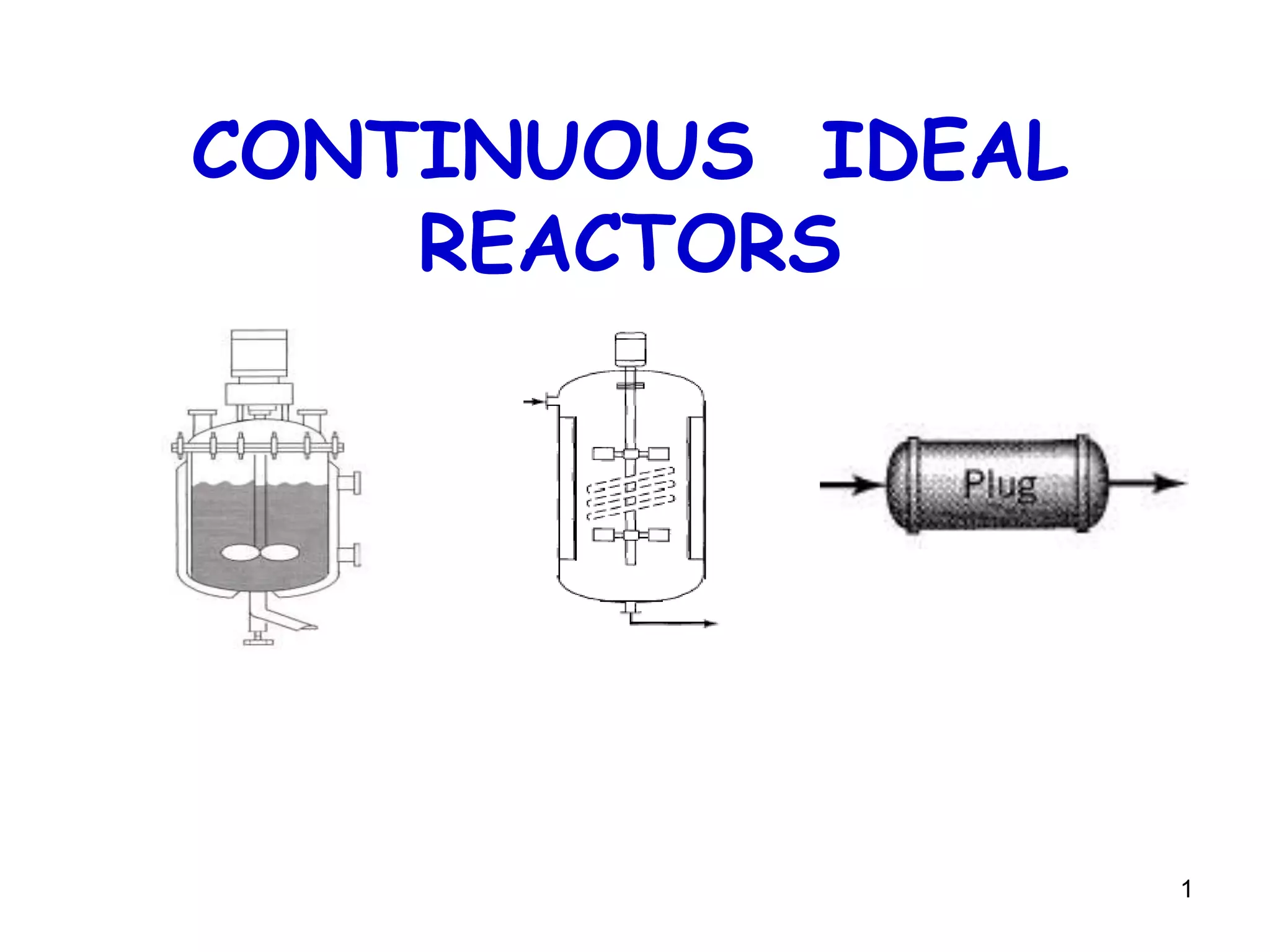
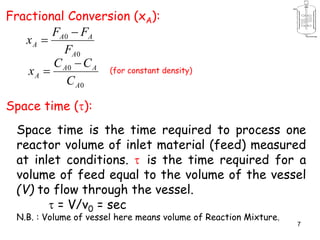
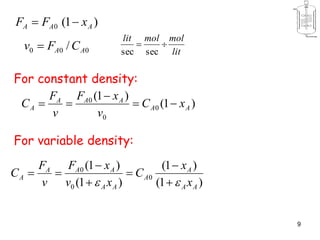
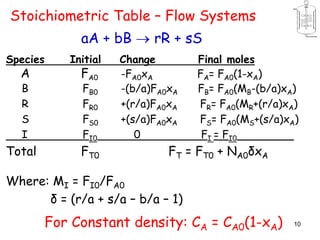
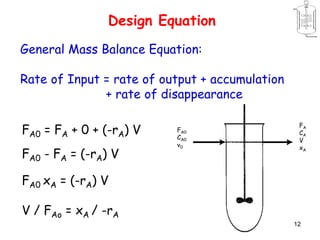
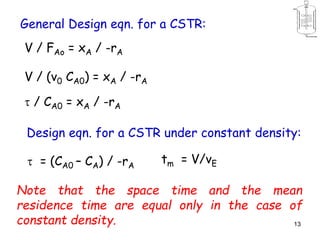
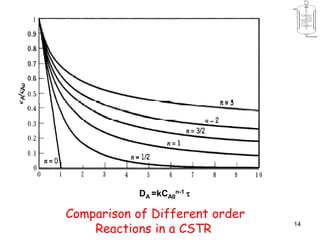
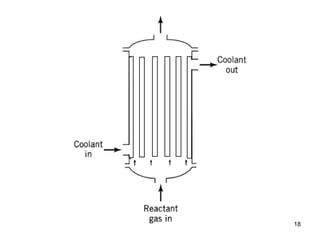
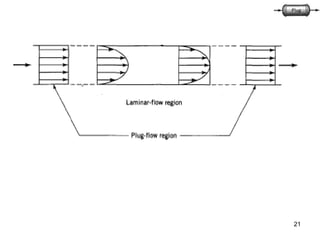
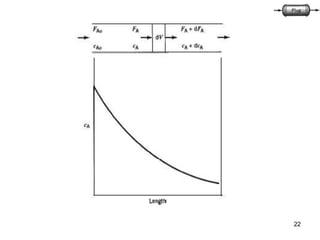
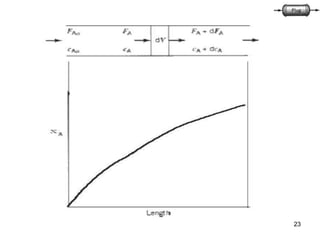
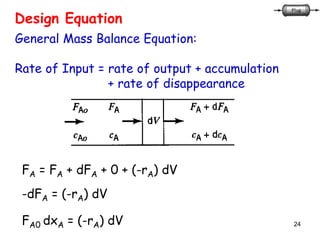
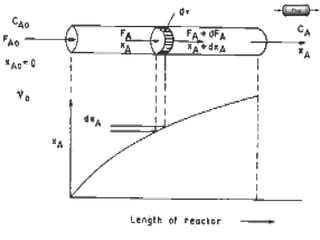
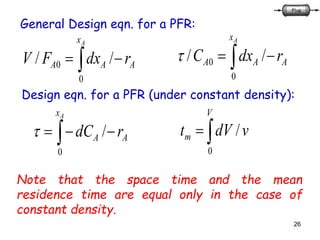
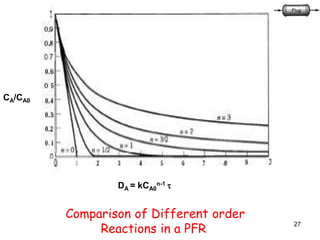
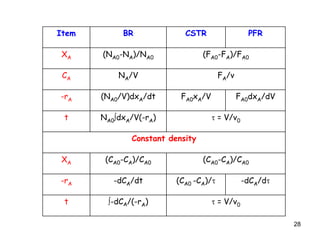
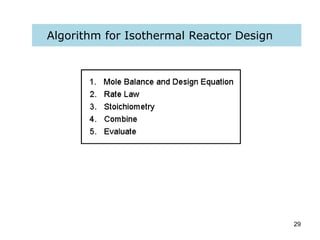
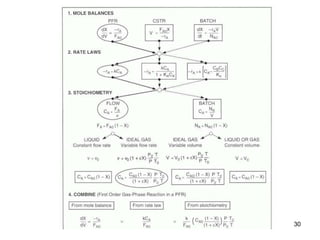
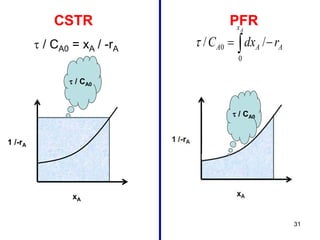
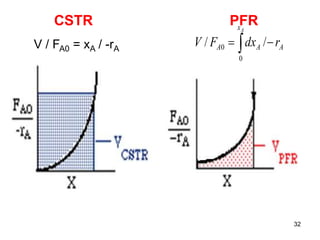
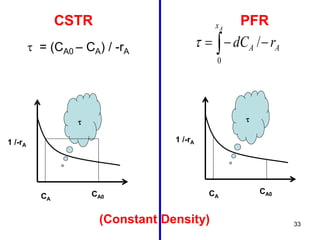
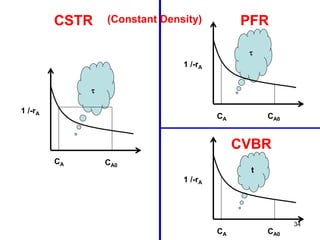
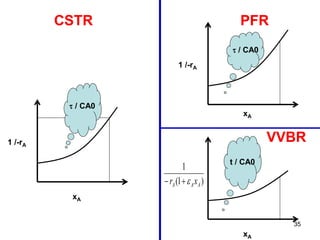
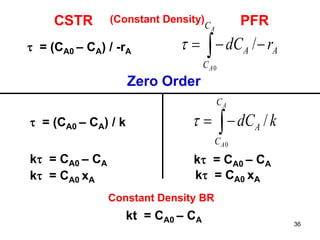
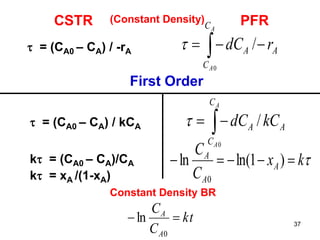
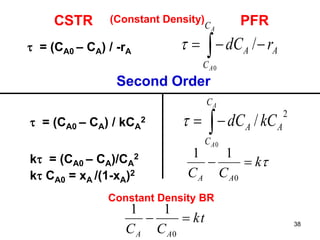
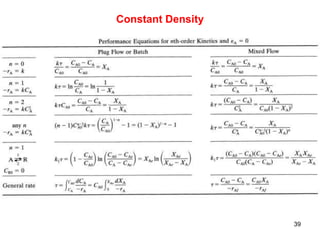
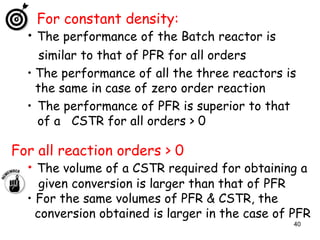
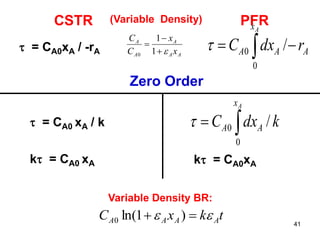
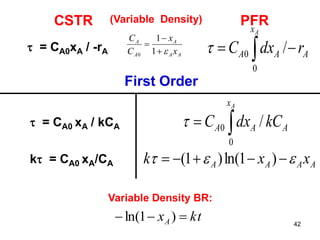
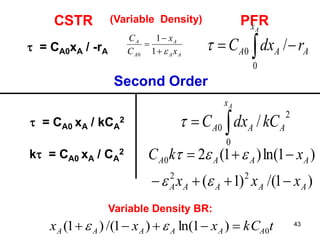
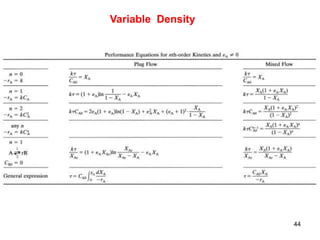
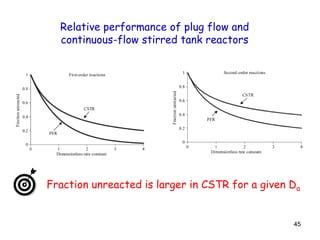
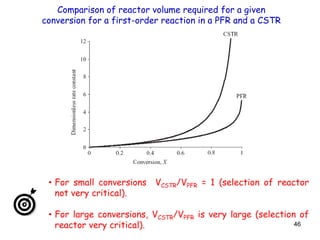
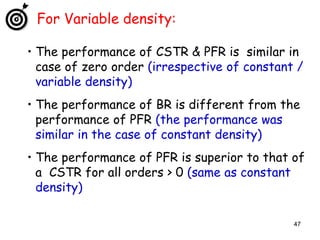
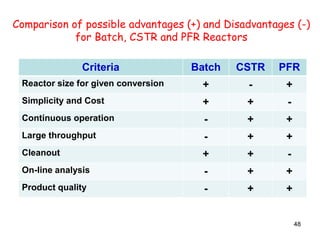
The document discusses continuous ideal reactors, focusing on Continuous Stirred Tank Reactors (CSTR) and Plug Flow Reactors (PFR). It outlines their operating principles, advantages, disadvantages, and performance comparisons under various conditions, emphasizing the differences in reactor design and conversion efficiency. Key parameters such as space time, mean residence time, and orders of reaction are also analyzed to highlight their influence on reactor performance.