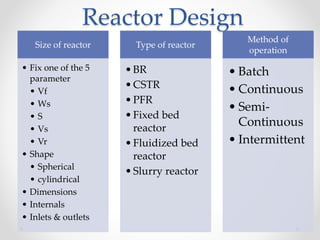
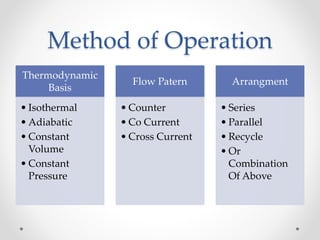
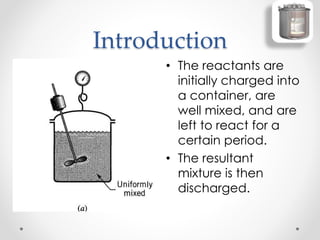
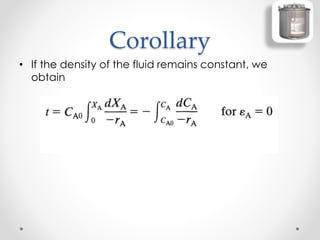
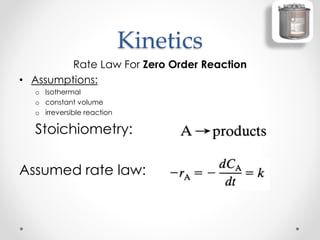
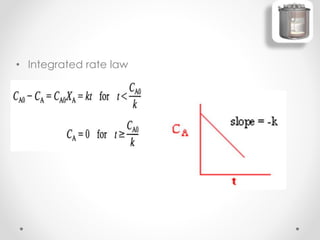
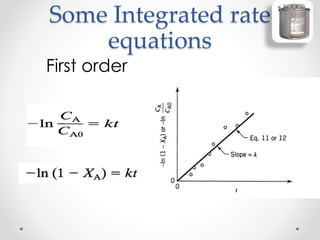
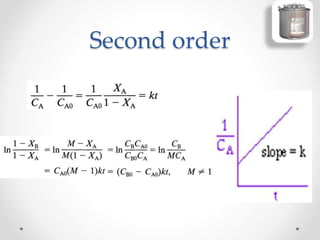
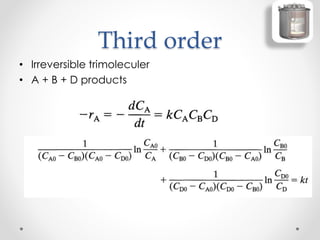
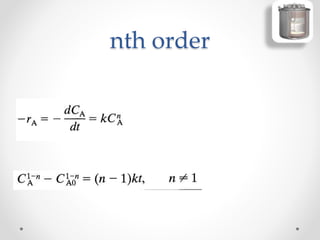
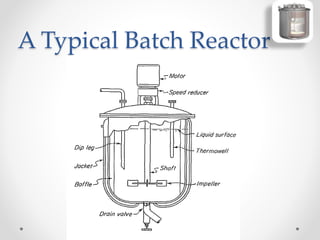
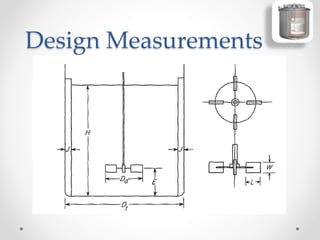
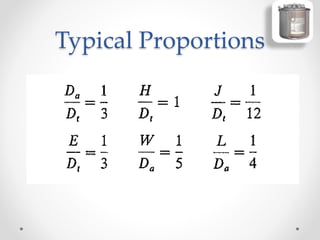
The document provides a comprehensive overview of batch reactors, including their working principles, advantages, disadvantages, and various design considerations. It discusses the importance of mean residence time, material, and energy balances, as well as the kinetics involved in batch processes. Additionally, it highlights applications suited for batch reactors, particularly in scenarios requiring small production quantities or during developmental stages.