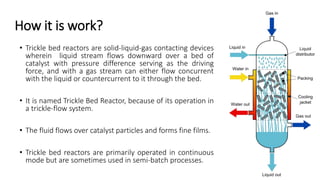
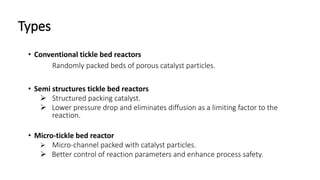
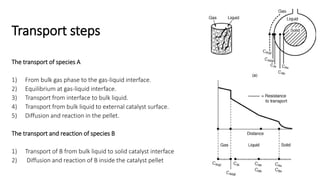
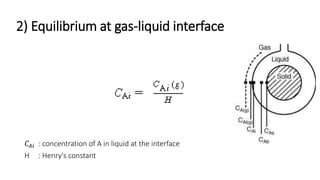
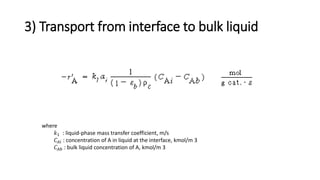
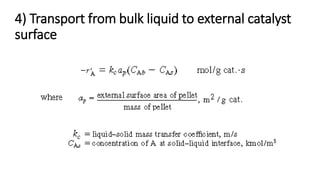
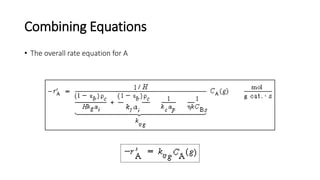
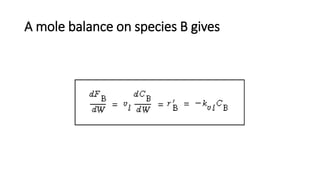
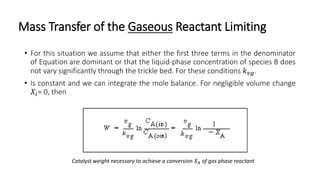
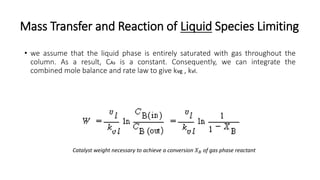
Trickle-bed reactors are solid-liquid-gas contacting devices where liquid flows downward over a packed bed of catalyst particles. Gas can flow concurrently or countercurrently through the bed. Liquid forms thin films over catalyst particles. Species transport and reaction involve multiple steps: from gas to liquid interface, interface to bulk liquid, bulk liquid to catalyst surface, diffusion within catalyst pellet. Trickle beds are useful for three-phase reactions like hydrodesulfurization but can develop hot spots or channeling.