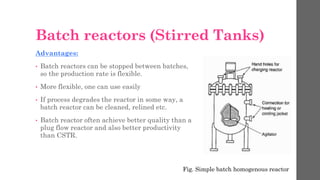
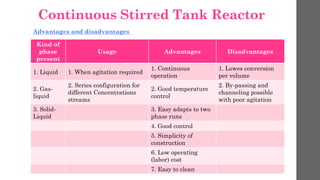
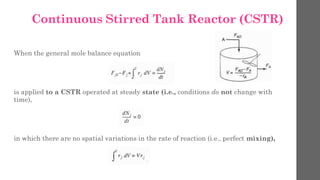
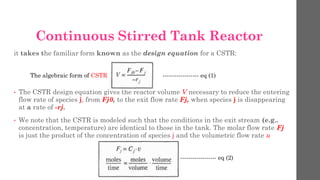
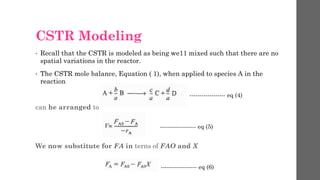
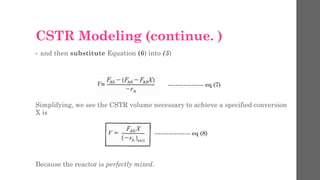
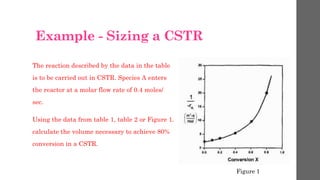
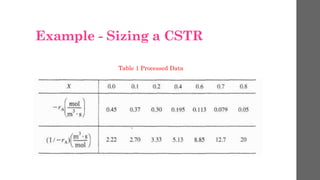
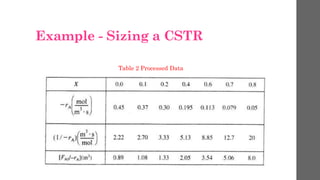
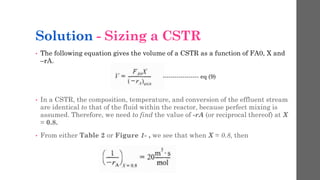
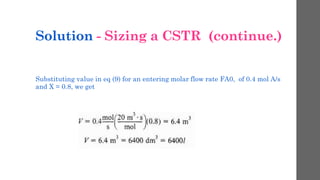
The document describes different types of reactors used in chemical processes. It discusses batch reactors, continuous stirred tank reactors (CSTR), plug flow reactors, fixed bed reactors, and fluidized bed reactors. For CSTRs, it provides the design equation that relates the reactor volume to the inlet and outlet flow rates of a reactant and the rate of reaction. It also gives an example of using this equation to calculate the volume of a CSTR needed to achieve 80% conversion of a reactant based on given kinetic data.