Organic Reactions Mechanisms
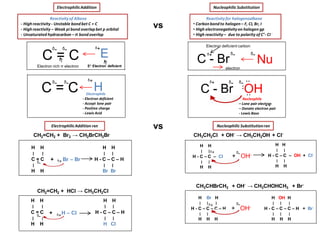
- 1. Electrophile - Electron deficient - Accept lone pair - Positive charge - Lewis Acid C - Br Reactivityfor halogenoalkane • Carbon bondto halogen – F, CI, Br, I • High electronegativityon halogen gp • High reactivity – due to polarity of C+ - CI - C - Br ᵟ+ ᵟ- electron Electron deficient carbon OH ..ᵟ-ᵟ+ Nucleophilic Substitutionrxn CH3CH2CI + OH- → CH3CH2OH + CI- H H ׀ ׀ H - C – C – CI ׀ ׀ H H + OH- ᵟ+ ᵟ- H H ׀ ׀ H - C – C – OH + CI- ׀ ׀ H H H Br H ׀ ׀ ׀ H - C – C – C – H ׀ ׀ ׀ H H H CH3CHBrCH3 + OH- → CH3CHOHCH3 + Br- + OH- H OH H ׀ ׀ ׀ H - C – C – C – H + Br- ׀ ׀ ׀ H H H ᵟ+ ᵟ- Nucleophilic SubstitutionElectrophilicAddition vs Reactivityof Alkene - High reactivity - Unstable bondbet C = C - High reactivity – Weak pi bond overlapbet p orbital - Unsaturated hydrocarbon – ᴨ bondoverlap C = C Electron rich π electron ᵟ- ᵟ- H ᵟ+ C = C ᵟ-ᵟ- E ᵟ+ E+ Electron deficient Nu ᵟ- ᵟ- Nucleophile – Lone pair electron – Donate electron pair - Lewis Base H H ׀ ׀ C = C ׀ ׀ H H CH2=CH2 + Br2 → CH2BrCH2Br + Br – Br ᵟ- ᵟ+ H H ׀ ׀ H - C – C – H ׀ ׀ Br Br vs CH2=CH2 + HCI → CH3CH2CI H H ׀ ׀ C = C ׀ ׀ H H ᵟ- + H – CIᵟ+ H H ׀ ׀ H - C – C – H ׀ ׀ H CI ElectrophilicAddition rxn
- 2. ᵟ- Electron rich region ElectrophilicSubstitutionrxn C6H6 + Br2 C6H5Br + HBr + Br-Br ᵟ+ + NO2 + ᵟ+ ElectrophilicSubstitution vs C = C Electron rich π electron ᵟ- ᵟ- ᵟ+ C = C ᵟ-ᵟ- E ᵟ+ E+ Electron deficient E ᵟ+ H H ׀ ׀ C = C ׀ ׀ H H CH2=CH2 + Br2 → CH2BrCH2Br + Br – Br ᵟ- ᵟ+ H H ׀ ׀ H - C – C – H ׀ ׀ Br Br vs CH2=CH2 + HCI → CH3CH2CI H H ׀ ׀ C = C ׀ ׀ H H ᵟ- + H – CIᵟ+ H H ׀ ׀ H - C – C – H ׀ ׀ H CI ElectrophilicAddition rxn E Electrophile - Electron deficient - Accept lone pair - Positive charge - Lewis Acid ᵟ++ H E + H Electron rich region H Br + HBr C6H6 + HNO3 C6H5NO2 + HCI AICI3 dry ether warm/conc H2SO4 H NO2 Reactivityof Alkene - High reactivity - Unstable bondbet C = C - High reactivity – Weak pi bond overlapbet p orbital - Unsaturated hydrocarbon – ᴨ bondoverlap Reactivityof Benzene (Unreactive) - Delocalization ofelectron in ring - Stabilitydue to delocalized π electron - Substitution instead of Addition C6H6 – no reaction with brown Br2(I) ethene decolourize brown Br2(I) Benzene –stable (unreactive) toward addition rxn Electrophile - Electron deficient - Accept lone pair - Positive charge - Lewis Acid H ElectrophilicAddition
- 3. Electrophile - Electron deficient - Accept lone pair - Positive charge - Lewis Acid C - Br OH ..ᵟ-ᵟ+ NucleophileElectrophile ᵟ+ C = C ᵟ- Nucleophile – Lone pair electron – Donate electron pair - Lewis Base Organic Rxn Addition rxn Substitution rxn Nucleophilic Substitution Free RadicalSubstitution ElectrophilicSubstitutionElectrophilicAddition rxn Free radicle CI CI CI CI. . : Radical (unpair electron) uv radiation H H ׀ ׀ C = C ׀ ׀ H H + Br – Br H H ׀ ׀ H - C – C – H ׀ ׀ Br Br ᵟ+ ᵟ- H H ׀ ׀ H - C – C – CI ׀ ׀ H H + OH- H H ׀ ׀ H - C – C – OH + CI- ׀ ׀ H H ᵟ-ᵟ+ H E+ + H Eᵟ+ H H ׀ ׀ C = C ׀ ׀ H H H H ׀ ׀ H - C – C – H ׀ ׀ CI CI H H ׀ ׀ H - C – C – H ׀ ׀ H CI H H ׀ ׀ H - C – C – H ׀ ׀ H OH Add HCI CI2 / UV H H ׀ ׀ H - C – C – CI ׀ ׀ H H H H ׀ ׀ H - C – C – OH + CI- ׀ ׀ H H H H ׀ ׀ H - C – C – NH2 + CI- ׀ ׀ H H H H ׀ ׀ H - C – C – CN + CI- ׀ ׀ H H NH3 OH- CN- H ׀ H - C – H ׀ H H ׀ H - C – CI + H ׀ H CI2 → 2 CI• CH3• + CI2 → CH3CI + CI• CI• + CH4 → HCI + CH3• H
- 4. Electrophile - Electron deficient - Accept lone pair - Positive charge - Lewis Acid C - Br OH ..ᵟ-ᵟ+ NucleophileElectrophile H ᵟ+ C = C ᵟ- Nucleophile – Lone pair electron – Donate electron pair - Lewis Base Free radicle CI CI CI CI. . : Radical (unpair electron) uv radiation H H ׀ ׀ C = C ׀ ׀ H H H H ׀ ׀ H - C – C – H ׀ ׀ CI CI H H ׀ ׀ H - C – C – H ׀ ׀ H CI H H ׀ ׀ H - C – C – H ׀ ׀ H OH Add HCI CI2 / UV H H ׀ ׀ H - C – C – CI ׀ ׀ H H H H ׀ ׀ H - C – C – OH + CI- ׀ ׀ H H H H ׀ ׀ H - C – C – NH2 + CI- ׀ ׀ H H H H ׀ ׀ H - C – C – CN + CI- ׀ ׀ H H NH3 OH- CN- H ׀ H - C – H ׀ H H ׀ H - C – CI + H ׀ H CI2 → 2 CI• CH3• + CI2 → CH3CI + CI• CI• + CH4 → HCI + CH3• Alkene – Addition rxn Halogenoalkane – Substitution rxn Alkane - Radical substitution H OH ׀ ׀ H - C – C – H ׀ ׀ H H H O ׀ ‖ H - C – C – H ׀ H H O ׀ ‖ H - C – C – OH ׀ H H O H ׀ ‖ ׀ H - C – C – C – H ׀ ׀ H H H OH H ׀ ׀ ׀ H - C – C – C – H ׀ ׀ ׀ H H H H OH H ׀ ׀ ׀ H - C – C – C – H ׀ ׀ ׀ H CH3 H Alcohol – Oxidation rxn 10 alcohol 20 alcohol 30 alcohol carboxylic acid aldehyde ketone no reaction
- 5. Electrophile - Electron deficient - Accept lone pair - Positive charge - Lewis Acid Reactivityof Alkene - High reactivity - Unstable bondbet C = C - High reactivity – Weak pi bond overlapbet p orbital - Unsaturated hydrocarbon – ᴨ bondoverlap C = C Electron rich π electron ᵟ- ᵟ- Br ᵟ+ H H ׀ ׀ C = C ׀ ׀ H H + H – Br ᵟ-ᵟ+ H H ׀ ׀ H - C – C – H ׀ + H ElectrophilicAddition SymmetricalAlkene HBr polar CH2=CH2 + HBr → CH3CH2Br : Br- H H ׀ ׀ H - C – C – H ׀ ׀ H Br CH2=CH2 + Br2 → CH2BrCH2Br Electrophilicaddition to symmetricalalkene H H ׀ ׀ C = C ׀ ׀ H H + Br – Br ᵟ+ ᵟ- Br2 non polar : induced dipole due to C=C H H ׀ ׀ H - C – C – H ׀ + Br : Br- H H ׀ ׀ H - C – C – H ׀ ׀ Br Br carbocation carbocation Heterolytic fission Heterolytic fission CH2=CH2 + Br2/H2O → CH2BrCH2Br H H ׀ ׀ C = C ׀ ׀ H H + Br – Br Heterolytic fission ᵟ+ ᵟ- H H ׀ ׀ H - C – C – H ׀ + Br : Br- H H ׀ ׀ H - C – C – H ׀ ׀ Br Br H H ׀ ׀ H - C – C – H ׀ + Br : OH- from H2O H H ׀ ׀ H - C – C – H ׀ ׀ Br OH H H ׀ ׀ C = C ׀ ׀ H H H H ׀ ׀ CH3 – C = C – CH3 + H – Br H H ׀ ׀ H - C – C – H ׀ ׀ H Br + H – Br H H ׀ ׀ CH3 – C – C – CH3 ׀ ׀ H Br only 1 product H CH3 ׀ ׀ H – C = C – H AsymmetricalAlkene + H – Br H CH3 ׀ ׀ H – C – C – H ׀ ׀ H Br H CH3 ׀ ׀ H – C – C – H ׀ ׀ Br H carbocation 2 product
- 6. H H ׀ ׀ C = C ׀ ׀ H H + H – Br ᵟ-ᵟ+ H H ׀ ׀ H - C – C – H ׀ + H HBr polar CH2=CH2 + HBr → CH3CH2Br : Br- H H ׀ ׀ H - C – C – H ׀ ׀ H Br CH2=CH2 + Br2 → CH2BrCH2Br Addition to symmetricalalkene H H ׀ ׀ C = C ׀ ׀ H H + Br – Br ᵟ+ ᵟ- Br2 non polar : induced dipole due to C=C H H ׀ ׀ H - C – C – H ׀ + Br : Br- H H ׀ ׀ H - C – C – H ׀ ׀ Br Br carbocation Heterolytic fission Heterolytic fission CH2=CH2 + Br2/H2O → CH2BrCH2Br H H ׀ ׀ C = C ׀ ׀ H H + Br – Br Heterolytic fission ᵟ+ ᵟ- H H ׀ ׀ H - C – C – H ׀ + Br : Br- H H ׀ ׀ H - C – C – H ׀ ׀ Br Br H H ׀ ׀ H - C – C – H ׀ + Br : OH- from H2O H H ׀ ׀ H - C – C – H ׀ ׀ Br OH H CH3 ׀ ׀ H – C = C – H + H – Br H CH3 ׀ ׀ H – C – C – H ׀ ׀ H Br H CH3 ׀ ׀ H – C – C – H ׀ ׀ Br H Addition to asymmetricalalkene CH2=CHCH3 + HBr → CH3CHBrCH3 or CH2BrCH2CH3 major minor CH3 CH3 ׀ ׀ H – C = C – CH3 + H – Br CH3 CH3 ׀ ׀ H – C – C – H ׀ ׀ H Br CH3 CH3 ׀ ׀ H – C – C – H ׀ ׀ Br H major minor ✓ ✓ Markovnokov rule - Hydrogen/electrophileadd to carbonwith most H2 bonded - Due to stable carbocationintermediateformed R ׀ R – C + ׀ R H ׀ R – C + ׀ R H ׀ H – C + ׀ R H ׀ H – C + ׀ H 30 carbocation > > > 20 carbocation 10 carbocation
- 7. H CH3 ׀ ׀ H – C ← C – H + ׀ H H CH3 ׀ ↓ H – C → C – H ׀ + H + H – Br ᵟ-ᵟ+ : Br- : Br- 2 alkyl gp – positive inductive effect – push electron to carbocation (more stable) Heterolytic fissionH CH3 ׀ ׀ H – C = C – H Addition to asymmetricalalkene CH2=CHCH3 + HBr → CH3CHBrCH3 major minor CH3 CH3 ׀ ׀ H – C = C–CH3 + H – Br CH3 CH3 ׀ ↓ H – C → C ← CH3 ׀ + H CH3 CH3 ↓ ׀ H – C ← C – CH3 + ׀ H major minor ✓ ✓ Markovnokov rule - H add to carbon with most H2 bonded - Due to stable carbocationformed R ׀ R – C + ׀ R H ׀ R – C + ׀ R H ׀ H – C + ׀ R > > H CH3 ׀ ׀ H – C – C – H ׀׀ H Br H CH3 ׀ ׀ H – C – C – H ׀׀ Br H 1 alkyl gp – positive inductive effect – push electron to carbocation (less stable) ᵟ+ ᵟ - : Br- CH3 CH3 ׀ ׀ H – C – C – CH3 ׀ ׀ H Br 3 alkyl gp – positive inductive effect – push electron to carbocation (more stable) : Br- 2 alkyl gp – positive inductive effect – push electron to carbocation (less stable) CH3 CH3 ׀ ׀ H – C – C – CH3 ׀ ׀ Br H 30 carbocation most stable 10 carbocation least stable H CH3 ׀ ↓ H – C → C – H ׀ + H H CH3 ׀ ׀ H – C ← C – H + ׀ H > 20 carbocation – greater positive inductive effect - more stable/lower charge density carbocation CH3 CH3 ׀ ↓ H – C → C ← CH3 ׀ + H > CH3 CH3 ↓ ׀ H – C ← C – CH3 + ׀ H 30 carbocation – greater positive inductive effect - more stable/lower charge density carbocation
- 8. H CH3 ׀ ׀ H – C ← C – H + ׀ Br H CH3 ׀ ↓ H – C → C – H ׀ + Br + Br – CI ᵟ-ᵟ+ : CI- : CI- 2 alkyl gp – positive inductive effect – push electron to carbocation (more stable) Heterolytic fissionH CH3 ׀ ׀ H – C = C – H Addition to asymmetricalalkene CH2=CHCH3 + BrCI → CH2BrCHCICH3 major minor CH3 CH3 ׀ ׀ H – C = C–CH3 + I – CI CH3 CH3 ׀ ↓ H – C → C ← CH3 ׀ + I CH3 CH3 ↓ ׀ H – C ← C – CH3 + ׀ I major minor ✓ ✓ Markovnokov rule - H add to carbon with most H2 bonded - Due to stable carbocationformed R ׀ R – C + ׀ R H ׀ R – C + ׀ R H ׀ H – C + ׀ R > > H CH3 ׀ ׀ H – C – C – H ׀׀ Br CI H CH3 ׀ ׀ H – C – C – H ׀׀ CI Br 1 alkyl gp – less positive inductive effect – (less stable) ᵟ+ ᵟ - : CI- CH3 CH3 ׀ ׀ H – C – C – CH3 ׀ ׀ I CI 3 alkyl gp – positive inductive effect – push electron to carbocation (more stable) : CI- 2 alkyl gp – less positive inductive effect – (less stable) CH3 CH3 ׀ ׀ H – C – C – CH3 ׀ ׀ CI I 30 carbocation most stable 10 carbocation least stable H CH3 ׀ ↓ H – C → C – H ׀ + Br H CH3 ׀ ׀ H – C ← C – H + ׀ Br > 20 carbocation – greater positive inductive effect - more stable/lower charge density carbocation CH3 CH3 ׀ ↓ H – C → C ← CH3 ׀ + I > CH3 CH3 ↓ ׀ H – C ← C – CH3 + ׀ I 30 carbocation – greater positive inductive effect - more stable/lower charge density carbocation EN CI higher EN CI higher
- 9. ᵟ- Electron rich region ElectrophilicSubstitutionrxn C6H6 + Br2 C6H5Br + HBr + Br-Br ᵟ+ + NO2 + ᵟ+ ElectrophilicSubstitutionElectrophilicAddition vs C = C Electron rich π electron ᵟ- ᵟ- ᵟ+ C = C ᵟ-ᵟ- E ᵟ+ E+ Electron deficient E ᵟ+ H H ׀ ׀ C = C ׀ ׀ H H CH2=CH2 + Br2 → CH2BrCH2Br + Br – Br ᵟ- ᵟ+ H H ׀ ׀ H - C – C – H ׀ ׀ Br Br vs CH2=CH2 + HCI → CH3CH2CI H H ׀ ׀ C = C ׀ ׀ H H ᵟ- + H – CIᵟ+ H H ׀ ׀ H - C – C – H ׀ ׀ H CI ElectrophilicAddition rxn E Electrophile - Electron deficient - Accept lone pair - Positive charge - Lewis Acid ᵟ++ H E + H Electron rich region H Br + HBr C6H6 + HNO3 C6H5NO2 + HCI AICI3 dry ether warm/Conc H2SO4 H NO2 Reactivityof Alkene - High reactivity - Unstable bondbet C = C - High reactivity – Weak pi bond overlapbet p orbital - Unsaturated hydrocarbon – ᴨ bondoverlap Reactivityof Benzene (Unreactive) - Delocalization ofelectron in ring - Stabilitydue to delocalized π electron - Substitution instead of Addition C6H6 – no reaction with brown Br2(I) ethene decolourize brown Br2(I) Benzene –stable (unreactive) toward addition rxn Electrophile - Electron deficient - Accept lone pair - Positive charge - Lewis Acid H
- 10. + Br – Br ᵟ-ᵟ+ Cyclohexene(Addition) vs Benzene (Substitution) ✓ Positive charge distributed in benzene ring (carbocation intermediate) Benzene highly unreactive to addition rxn : Br- + + Br – Br + Br – Br AICI3 dry ether Benzene undergo substitution rxn Cyclohexene undergo addition rxn Benzene undergo electrophilic substitution (Bromination) Loss H+ enable aromatic ring to reform Br - Br Br Br Benzene undergo electrophilic substitution (Nitration) +NO2 NO2 NO2 Positive charge distributed in benzene ring (carbocation intermediate) Loss H+ enable aromatic ring to reform C6H6 + HNO3 C6H5NO2 Conc H2SO4 50C Conc HNO3 + H2SO4 produce NO2 + electrophile + H Reactivityof Benzene (Unreactive) - Delocalization ofelectron in ring - Stabilitydue to delocalized π electron - Substitution instead of Addition ElectrophilicSubstitution H E ᵟ+ + + H E Electron rich region C6H6 + Br2 C6H5Br + HBr AICI3 dry ether H + Br-Br ᵟ+ + HBr Br ✓
- 11. OH O ׀ ‖ CH3-C–CH3 + [O] CH3- C– CH3 H ׀ CH3-CH2-OH + [O] CH3- C = O Reduction rxnOxidation rxn MnO4 - /H+ K2Cr2O7/H+ 10 Alcohol – Oxidised to Aldehyde and Carboxylic acid 20 Alcohol - Oxidised to Ketone MnO4 - /H+ K2Cr2O7/H+ MnO4 - /H+ K2Cr2O7/H+ Oxidation vs Reduction rxn O ‖ CH3-COH Oxidation of Alcohol Acidified dichromate(VI)/permanganate(VII) Reduction carbonyl (C = O) Sodium borohydride (NaBH4) Lithium aluminium hydride (LiAIH4) / dry ether O ‖ CH3-COH CH3CHO CH3CH2OH O OH ‖ ׀ CH3-C–CH3 CH3- C– CH3 [H-] [H-] NaBH4 NaBH4 Carboxylic acid reduced to aldehyde / alcohol NaBH4 [H-] Ketone reduced to alcohol Hydride ion (nucleophile) :H- produce hydride ion / :H- O ‖ CH3-COH CH3CH2OH Carboxylic acid reduced alcohol [H-] LiAIH4 dry ether with acid stronger reducing agent Sn / conc HCI / reflux Nitrobenzene reduced to phenylamine NH3 +NO2 NH2 NaOH phenylammonium ion reducing agent Convert benzene to phenylamine Convert propanoic acid to propanol Convert ethanal to ethanol O ‖ CH3CH2 COH CH3CH2CH2OH [H-] stronger reducing agent LiAIH4 dry ether / acid CH3CHO CH3CH2OH [H-] NaBH4 50C NO2 conc HNO3 + H2SO4 Sn / conc HCI / reflux NH3 + NaOH NH2
- 12. ׀ ׀ C - C –OH ׀ ׀ O ‖ C – C – C O ‖ C – C – H O ‖ C – C – OH O ‖ C –C – C– O – C – C No reaction 1o alcohol [O]/Cr2O7/H+ Aldehyde Ketone Carboxylic Acid Free radical substitution CI2/ UV Halogenoalkane Alkane 2o alcohol [O]/ Cr2O7/H+ [O]/ Cr2O7/H+ 3o alcohol [O]/ Cr2O7/H+ Substitution warm / OH- Alcohol Alcohol Alkene Elimination 100C /Conc alcoholic OH- Alkane Halogenoalkane Dihalogenoalkane Condensation Ester Addition Polymerisation X ׀ ׀ C – C – CI ׀ ׀ ׀ ׀ C = C ׀ ׀ ׀ ׀ ׀ ׀ C – C – C – C ׀ ׀ ׀ ׀ ׀ ׀ C – C ׀ ׀ H CI ׀ ׀ C – C ׀ ׀ CI CI ׀ ׀ C – C ׀ ׀ Br Br ׀ ׀ C – C ׀ ׀ ׀ ׀ C – C – OH ׀ ׀ Start here PolyAlkene ׀ ׀ C – C ׀ ׀ H H [H]/ NaBH4[H]/ NaBH4 [H]/ NaBH4 oxidation reduction oxidation oxidation reduction reduction conc HNO3 / H2SO4 50C NO2 Sn / conc HCI / reflux NH3 + NaOH NH2
- 13. C – C = C – C → C – C – C – C ‖ O Synthetic routes C –C –C – I → C – C – C-H ‖ O Two steps 1 - Addition of H2O 2 - Oxidation alcohol to ketone Two steps 1– Substitution with OH- to alcohol 2- Oxidation alcohol to aldehyde But-2-ene to Butanone 1-iodopropane to propanal 1-chloropropane to propanoic acid C –C –C –CI → C – C–COOH Three steps 1 – Substitution with OH- to alcohol 2- Oxidation alcohol to aldehyde 3 - Oxidation aldehyde to carboxylic acid C – C = C – C C – C – C – C ׀ ׀ H OH C – C – C – C ‖ O H2O /300C H2SO4 catalyst [O] oxidation K2Cr2O7/H+ C – C – C – I C – C – C – H ‖ O C – C – C–OH warm NaOH SN2 [O] oxidation K2Cr2O7/H+ C – C – C – CI C – C – COOH C – C – C–OH C – C – C – H ‖ O warm NaOH SN2 [O] oxidation K2Cr2O7/H+ Propane to propanoic acid C –C –C → C –C –COOH C – C – C C – C – COOH C – C – C–CI C – C – C–OH [O] oxidation K2Cr2O7/H+ reflux Warm NaOH SN2 Free radical substitution UV / CI2 Three steps 1 – Free radical substitution to halogenoalkane 2– Substitution with OH- to alcohol 3 – Oxidation alcohol to carboxylic acid [O] oxidation K2Cr2O7/H+ reflux
- 14. Synthetic routes Propane to propyl propanoate Butene to butanone Three steps 1 – Addition HBr 2– Substitution with OH – 3 – Oxidation of alcohol to ketone Four steps 1 – Free radical substitution/UV 2– Substitution with OH- 3 – Oxidation alcohol to carboxylic acid 4 – Esterification with conc acid Ethene to ethanoic acid C – C ׀ ׀ H OH C – C – H ‖ O C – COOH Three steps 1 – Addition using H2O 2- Oxidation alcohol to aldehyde 3 – Oxidation aldehyde to carboxylic acid Ethanol to ethyl ethanoate C – C-OH → C–COO–C–C C – C – O – C – C ‖ O C–C–OH C – COOH Esterification Ethanol + ethanoic acid Conc H2SO4 Two steps 1 – Oxidation alcohol to carboxylic acid 2– Esterification with ethanol/conc acid C = C → C – COOH Free radical substitution UV / CI2 C–C–C–CI C – C – C C–C–C–OH Warm NaOH SN2 C–C–COOH [O] oxidation/reflux K2Cr2O7/H+ C – C – C – O – C – C – C ‖ O Esterification Propanol + propanoic acid Conc H2SO4 C – C = C – C C – C – C – C ‖ OAddition HBr C – C – C – C ׀ ׀ Br H Warm NaOH SN2 C – C – C – C ׀ ׀ OH H [O] oxidation K2Cr2O7/H+ [O] oxidation K2Cr2O7/H+ reflux C – C = C – C → C – C – C – C ‖ O C = C H2O /300C H2SO4 catalyst [O] oxidation K2Cr2O7/H+ [O] oxidation K2Cr2O7/H+ reflux C – C – C → C – C – C –O–C–C–C ‖ O
- 15. Synthetic routes Benzene to phenylamine Ethanoic acid to ethyl ethanoate Two steps 1 – Reduction to alcohol 2– Esterification with ethanoic acid/conc acid C – COOH C–C–OH Three steps 1 – Nitration substitution of benzene 2– Reduction of nitrobenzene 3 – Addition NaOH Ethanoic acid to ethanol C – C ׀ ׀ H OH C – C – H ‖ O C – COOH Two steps 1 – Reduction acid to aldehyde 2- Reduction aldehyde to alcohol C – COOH → C –COO–C–C C – C – O – C – C ‖ O Reduction [H-] LiAIH4 dry ether acid Esterification Ethanol + ethanoic acid Conc H2SO4 Ethane to Ethanol C – C → C–C-OH C – C C – C – CI Two steps 1 – Free radical substitution/UV 2– Substitution with OH- NO2 NH2 NH2 NH3 + conc HNO3 conc H2SO4 50C Sn / conc HCI / reflux NaOH C – COOH → C – C-OH Reduction [H-] NaBH4 Reduction [H-] NaBH4 Free radical substitution UV / CI2 C – C ׀ ׀ H OH warm NaOH SN2
