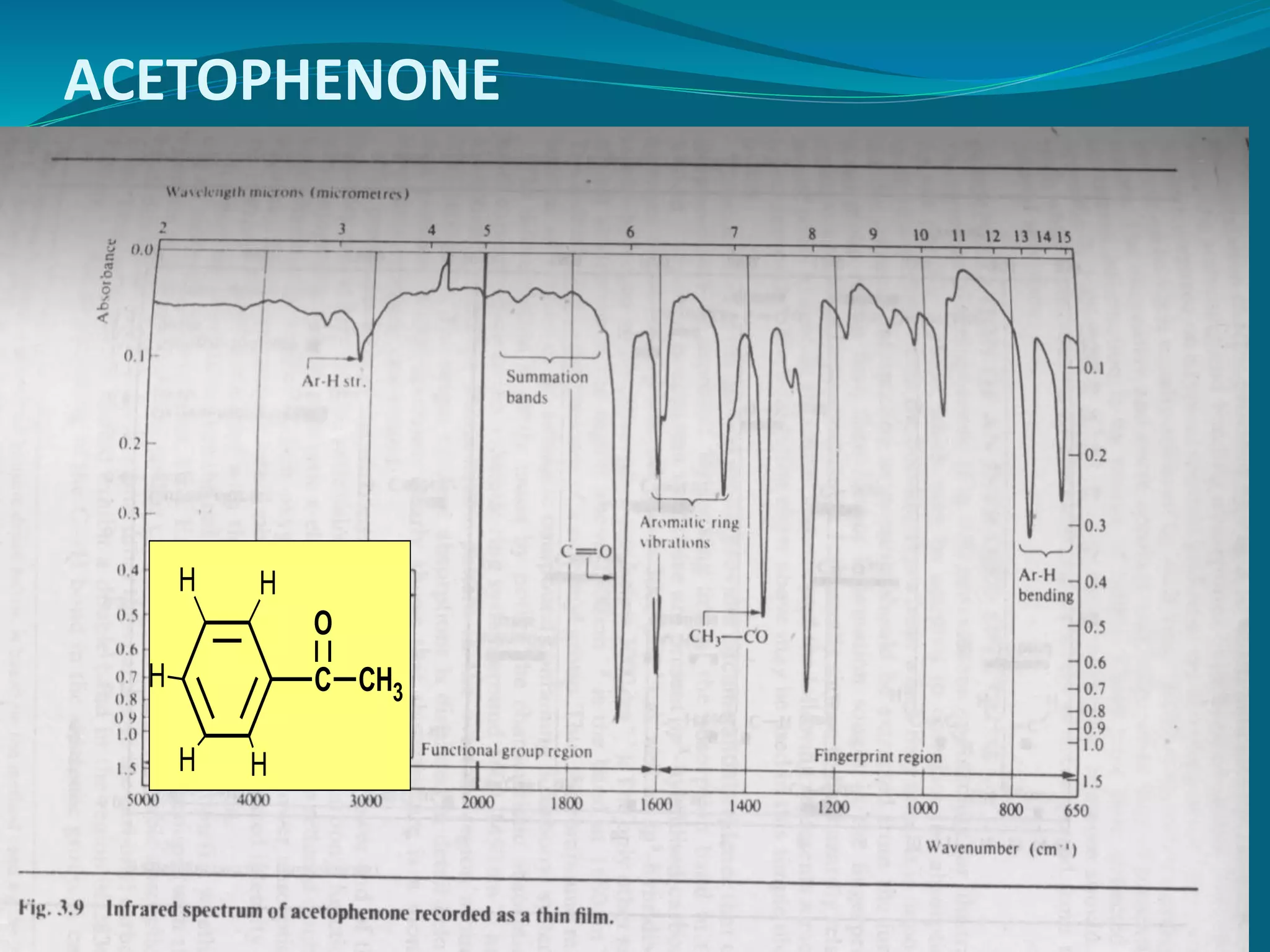
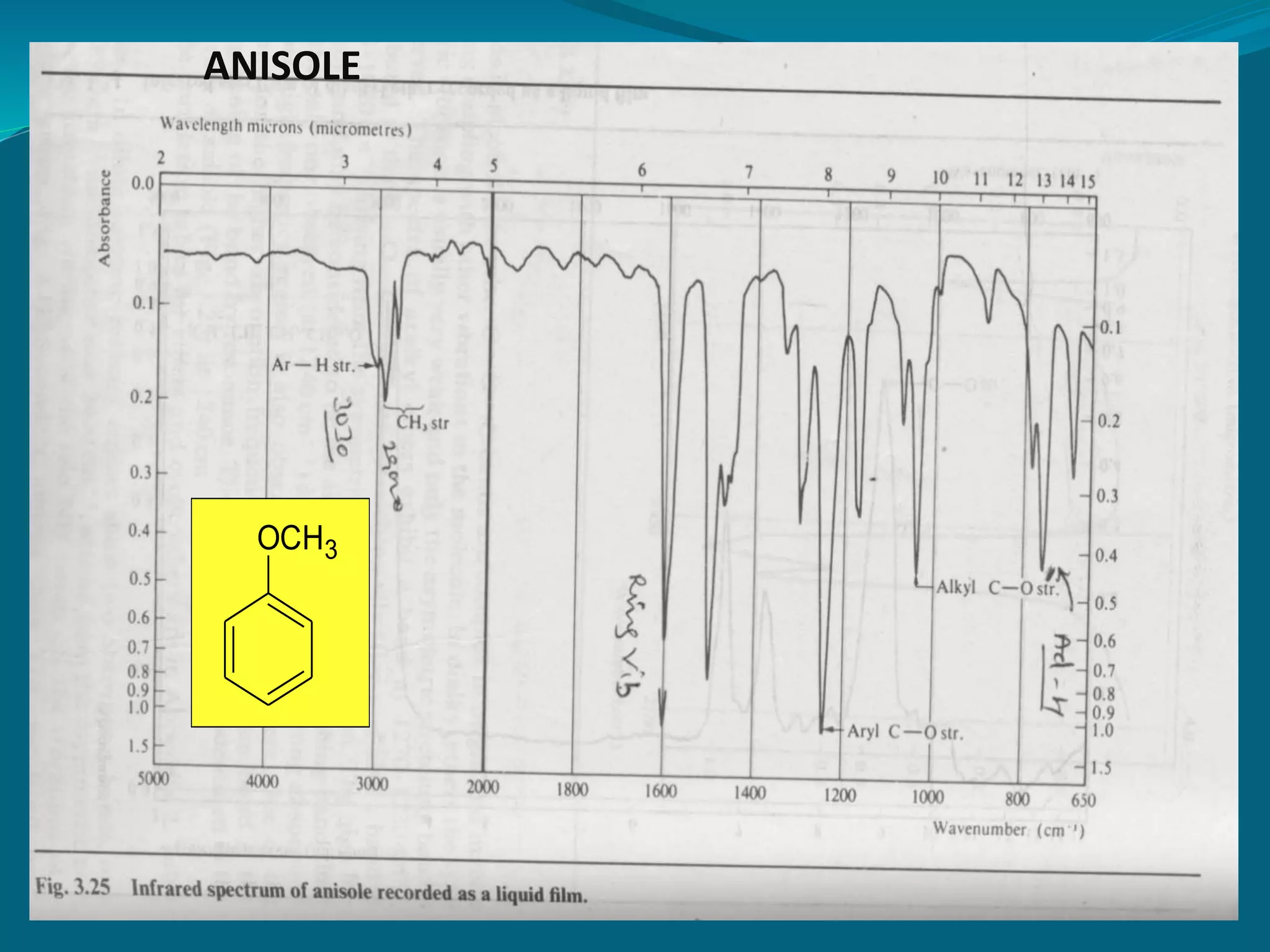
This document discusses the application of infrared spectroscopy in research. It begins by introducing the electromagnetic spectrum and infrared region. It then covers the principles of IR spectroscopy, including how molecular vibrations can be observed in IR spectra. Factors that determine peak positions, intensities, and widths are explained. Common vibrational modes like stretching and bending are described. The document discusses interpreting IR spectra for organic compounds, including distinguishing functional groups and molecular structure. It also covers practical aspects, applications in various fields like pharmaceutical research and quality control, and limitations of IR analysis.