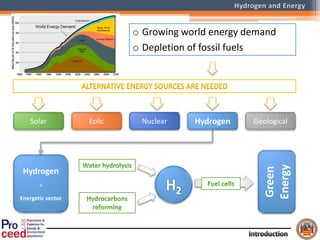
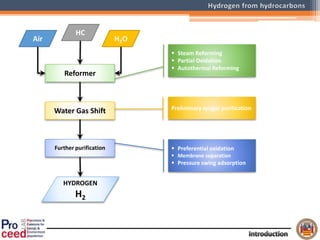
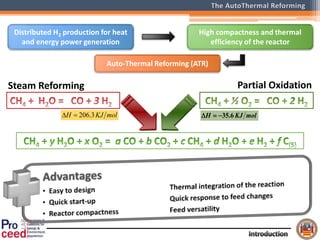
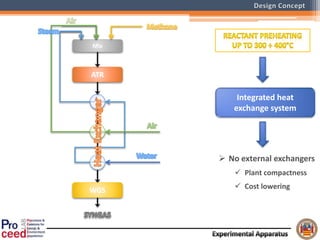
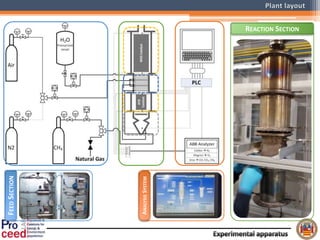
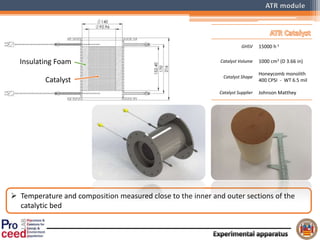
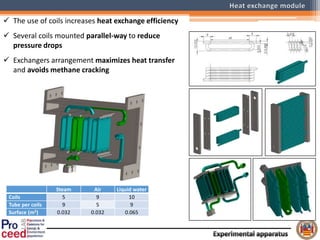
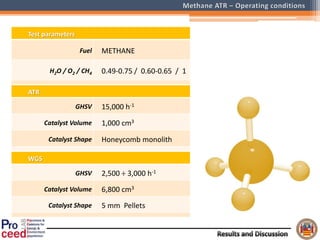
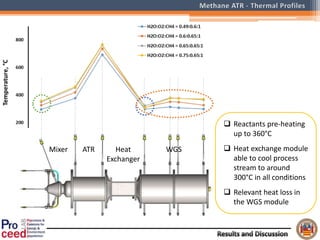
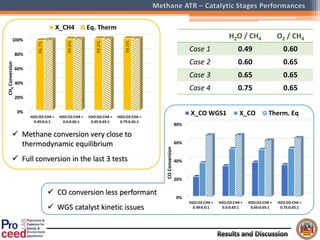
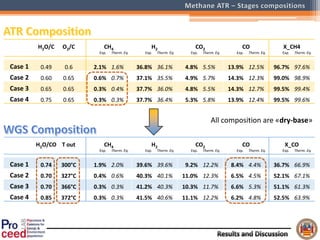
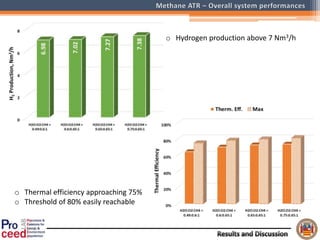
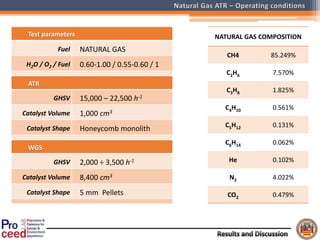
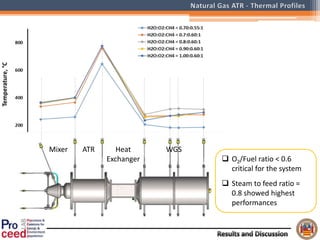
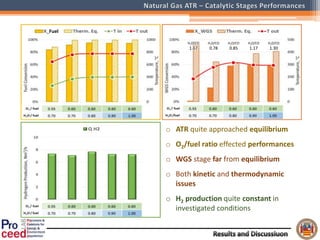
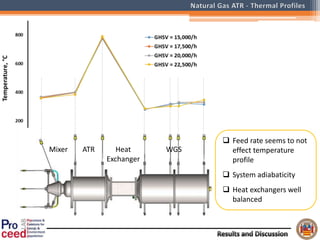
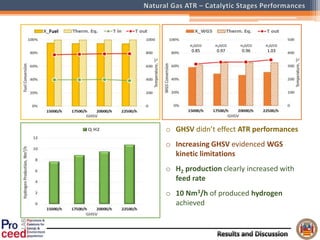
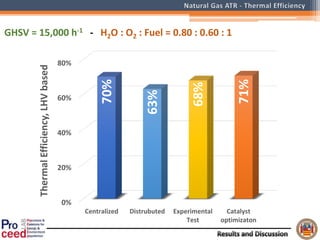
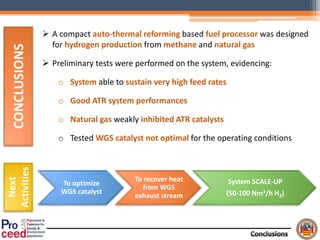
A compact auto-thermal reforming (ATR) based fuel processor was designed to produce 10 Nm3/h of hydrogen from methane and natural gas. Preliminary tests showed the ATR system could sustain high feed rates and natural gas was only weakly inhibited. The water-gas shift (WGS) catalyst tested was not optimal as it performed far from equilibrium and limited carbon monoxide conversion. Further work is needed to optimize the WGS catalyst, recover heat from the WGS exhaust, scale up the system to 50-100 Nm3/h of hydrogen production.