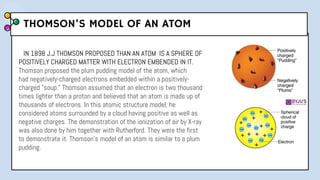
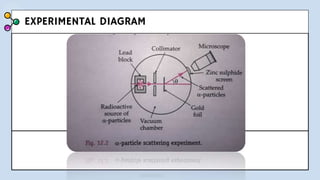
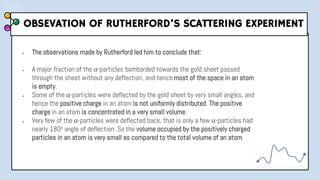
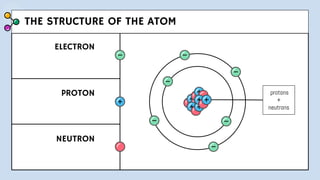
1) The document discusses Thomson's plum pudding model of the atom and its limitations. It then describes Rutherford's alpha scattering experiment, the conclusions he drew, and his nuclear model of the atom.
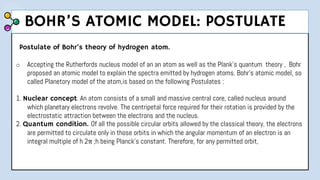
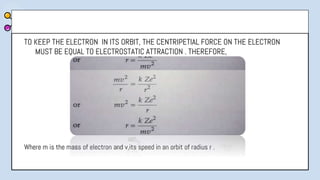
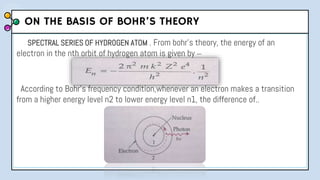
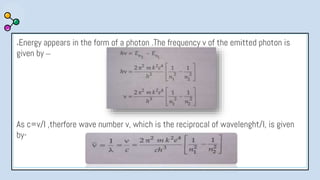
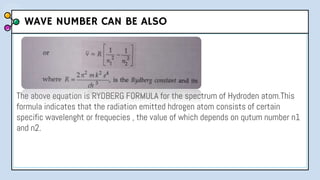
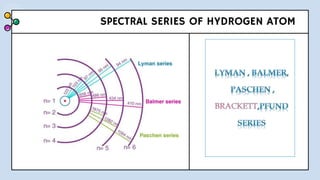
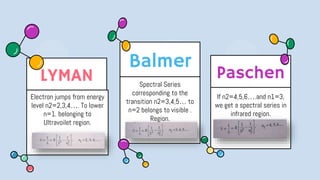
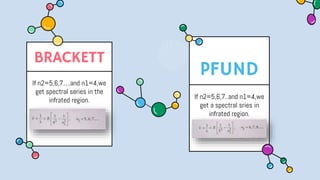
2) Bohr's model improved upon Rutherford's by proposing quantized electron orbits and energy levels based on Planck's quantum theory. This allowed Bohr to explain the hydrogen spectral series.
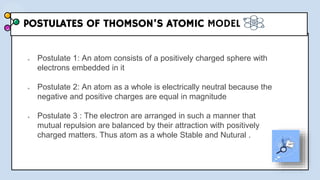
3) The document provides details of Thomson, Rutherford, and Bohr's atomic models, including their postulates and limitations. It discusses key experiments and conclusions that improved understanding of atomic structure over time.