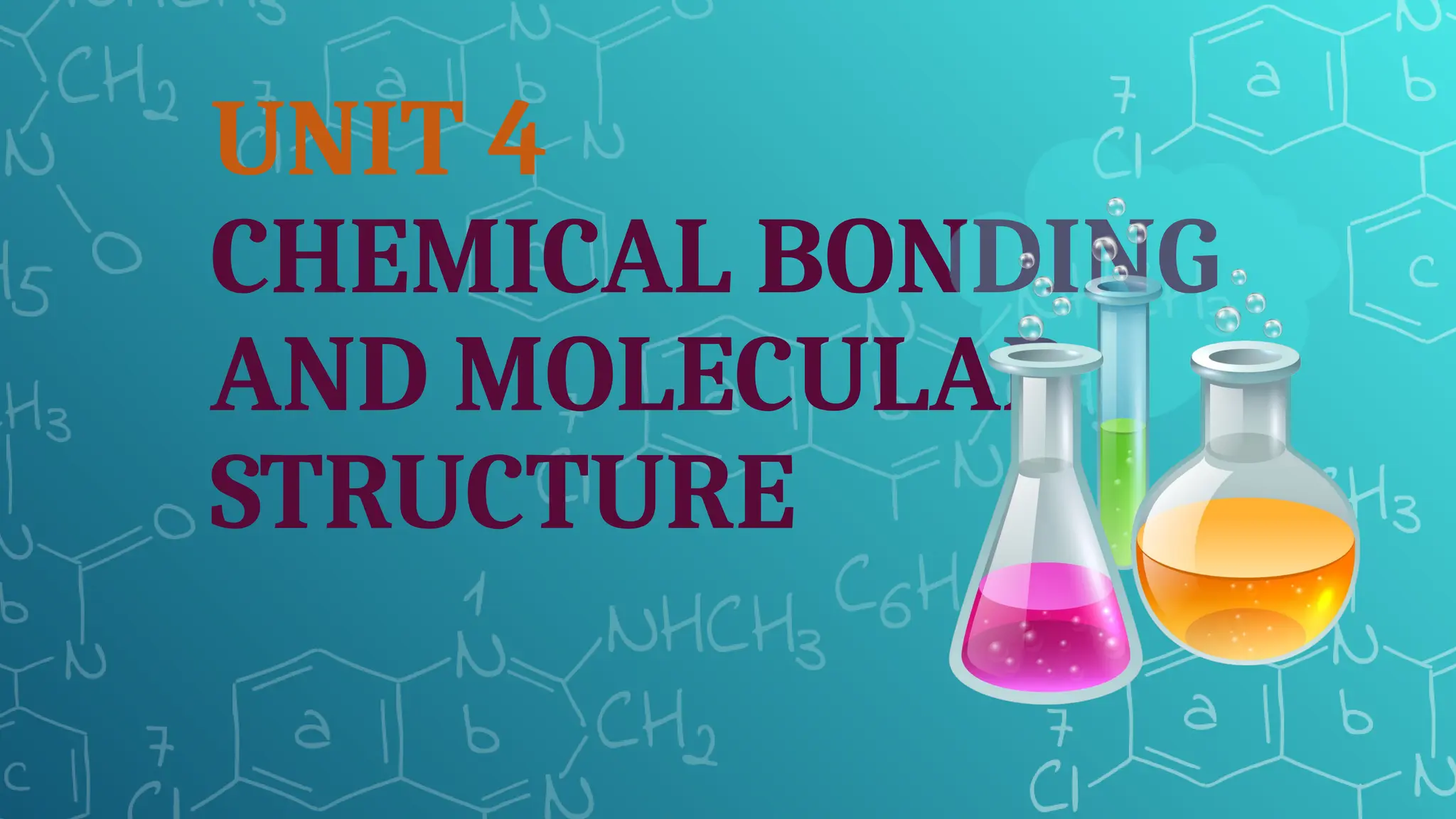
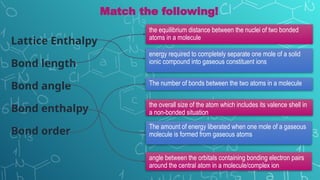
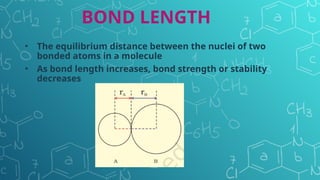
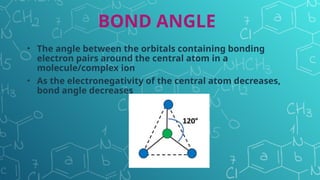
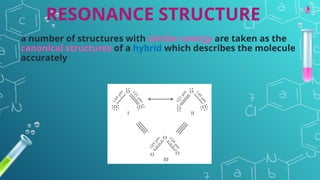
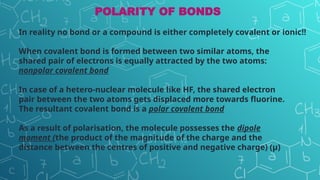
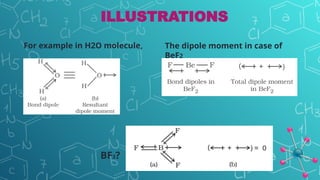
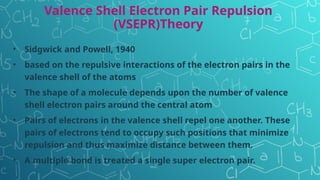
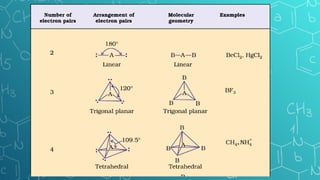
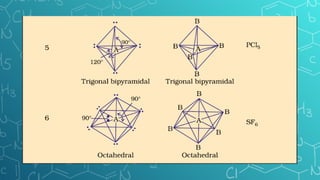
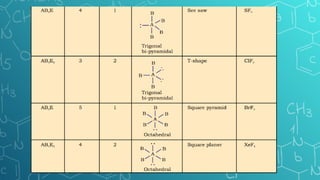
The document discusses chemical bonding and molecular structure, covering concepts such as lattice enthalpy, bond length, bond angle, bond enthalpy, and bond order. It explains resonance structures, polarity of bonds, dipole moments, and Fajan's rules, as well as the VSEPR theory for predicting molecular shapes based on electron pair repulsion. Key features of these concepts are summarized, including how bond characteristics affect molecular stability and polarity.