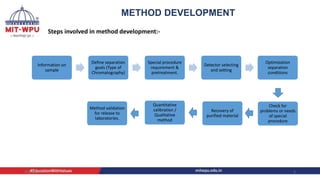
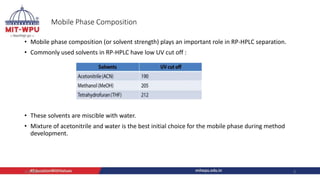
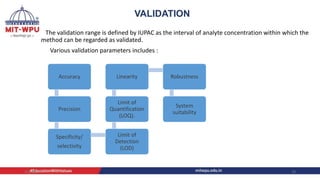
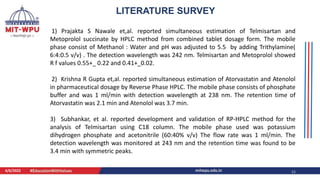
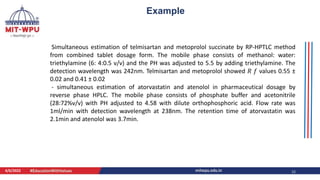
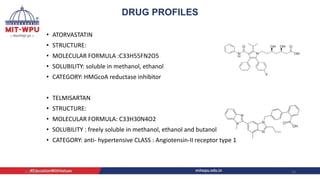
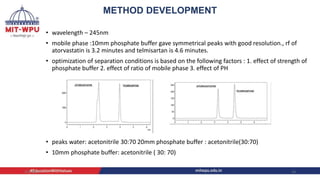
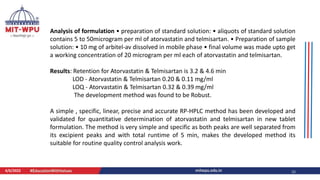
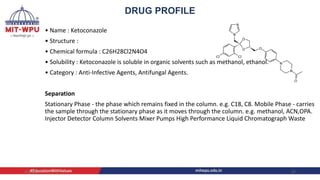
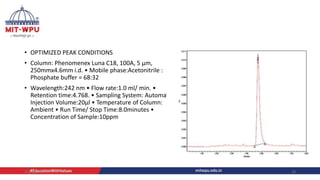
Nilesh Dashrath Kamble presented a seminar on method development and validation in HPLC. The presentation discussed the steps involved in HPLC method development including column selection, mobile phase composition, pH range selection, and optimization of separation conditions. It also covered validation parameters such as accuracy, precision, specificity, limit of detection, and limit of quantification as per ICH guidelines. The presentation included an example method development for the simultaneous estimation of atorvastatin and telmisartan from a tablet formulation.