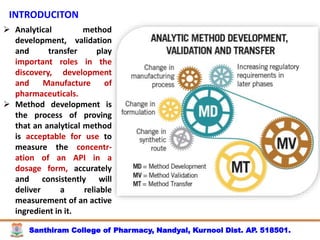
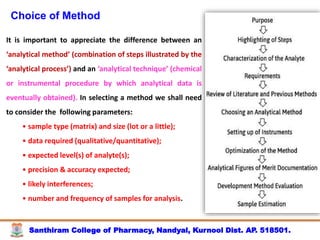
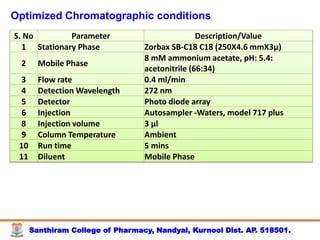
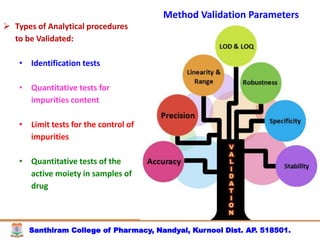
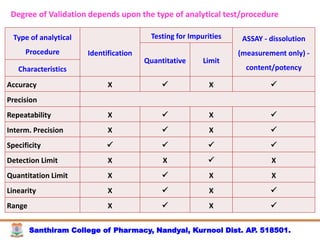
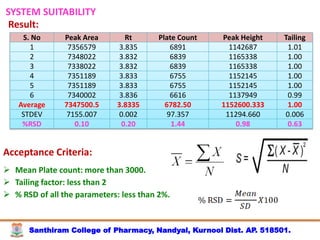
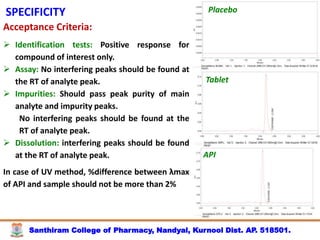
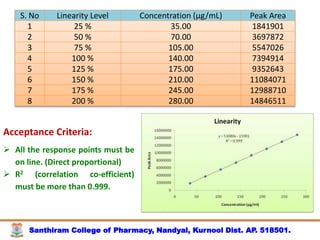
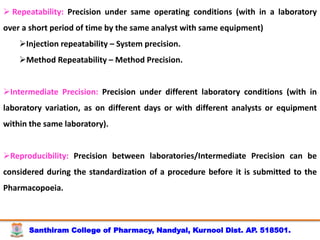
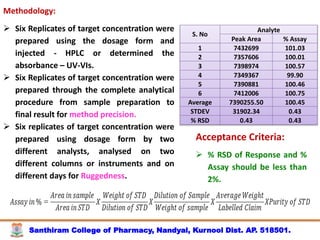
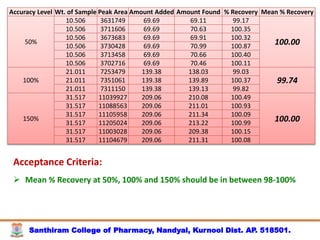
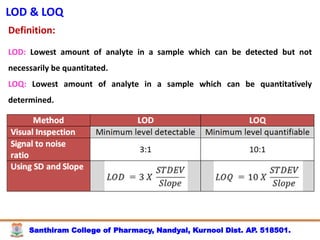
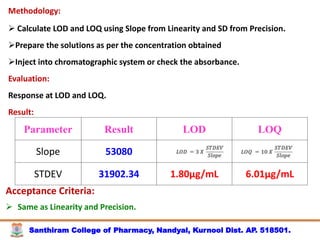
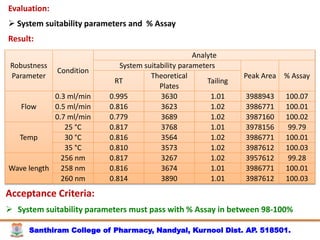
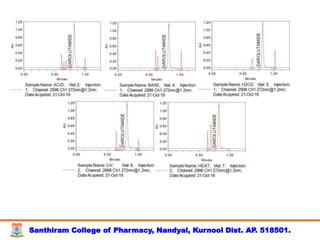
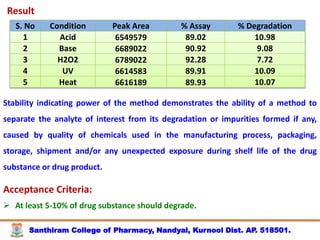
The document discusses analytical method development, validation and transfer. It begins by introducing the importance of method development, validation and transfer in pharmaceutical analysis. It then discusses some key aspects of each including the objectives of method development, definition of validation, and the purpose of method transfer. The document provides examples of parameters to consider for method development including sample type, required data, analyte levels, and expected precision and accuracy. It also gives an overview of common validation parameters like accuracy, precision, specificity, range and linearity. The document aims to provide guidance on establishing reliable analytical methods for pharmaceutical applications.