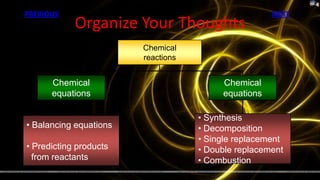
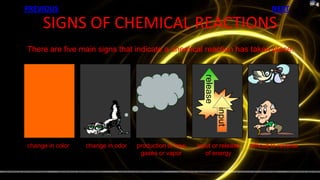
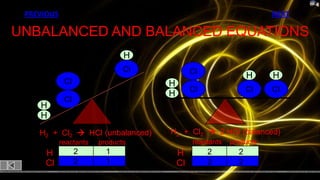
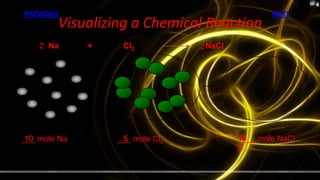
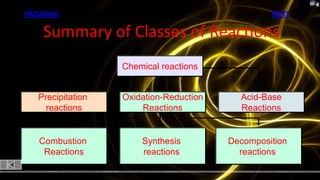
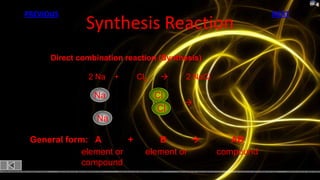
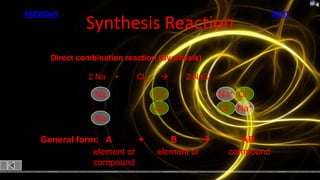
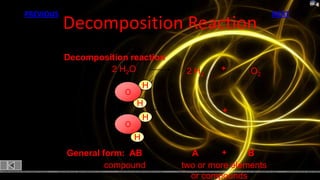
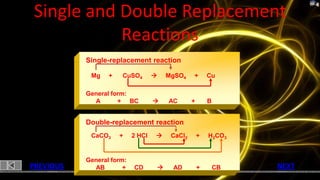
This document provides information about chemical reactions and equations. It discusses classifying reactions by type, writing balanced and unbalanced equations, predicting products from reactants, and identifying signs of chemical reactions such as gas production or color changes. Key reaction types covered are synthesis, decomposition, single and double displacement reactions, as well as oxidation-reduction reactions. The document also discusses writing word equations, balancing equations, and identifying reactants and products.