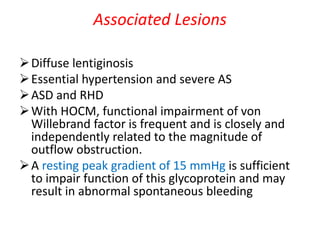
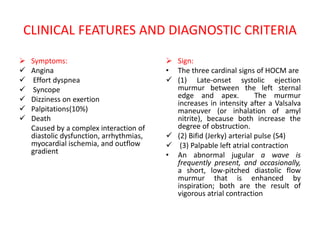
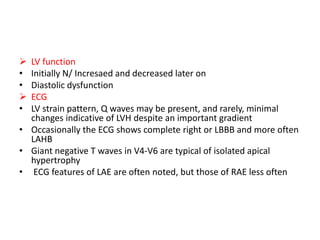
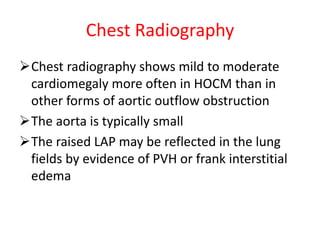
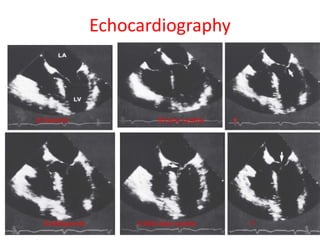
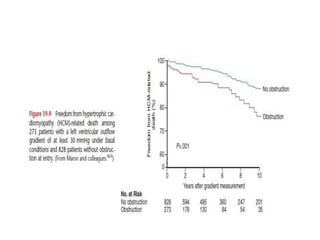
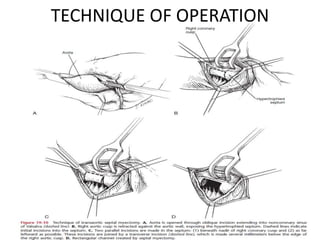
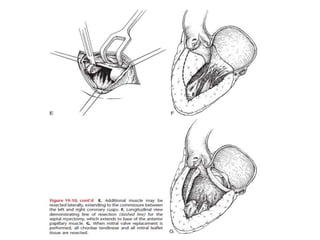
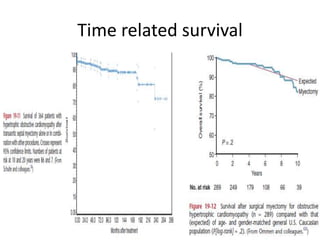
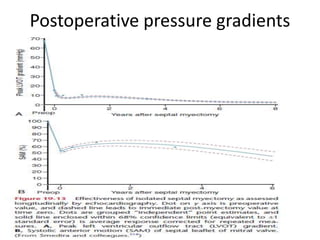
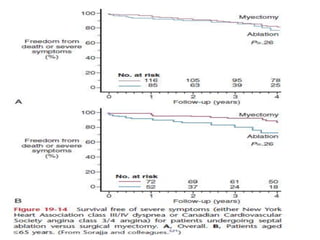
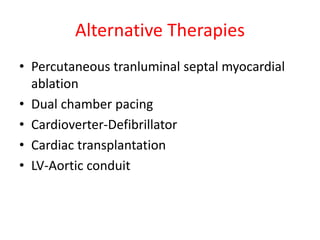
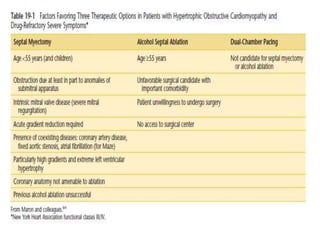
Hypertrophic obstructive cardiomyopathy (HOCM) is a genetic heart condition characterized by thickened heart muscle and reduced space in the left ventricular outflow tract. It is caused by mutations in sarcomere proteins. Symptoms include chest pain, dizziness, and heart failure. Diagnosis is made through echocardiogram showing septal hypertrophy and left ventricular outflow tract obstruction. Septal myectomy surgery aims to relieve outflow tract obstruction by removing hypertrophic septal tissue. While early mortality risk is high, long term survival is good for most patients after surgery. Residual systolic anterior motion of the mitral valve and gradients remain issues.