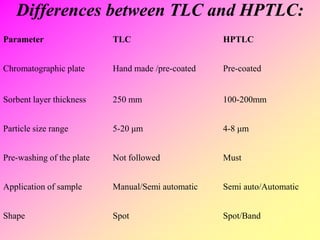
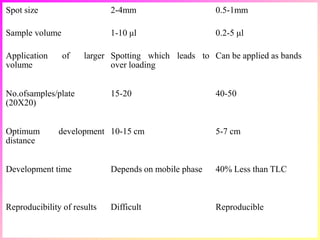
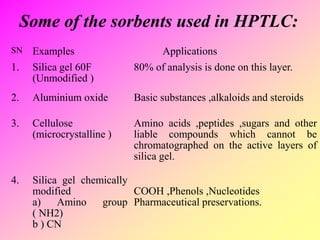
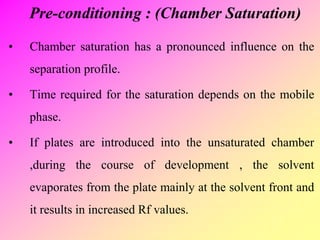
High-performance thin-layer chromatography (HPTLC) is an advanced method of planar chromatography that enhances the traditional thin-layer chromatography (TLC) technique by optimizing various parameters. HPTLC involves the use of pre-coated plates, a well-defined mobile phase, and automated application methods, leading to improved reproducibility and efficiency in separating and quantifying compounds. Applications of HPTLC span across pharmaceutical quality control, food analysis, and forensic investigations, utilizing specific detection methods for qualitative and quantitative analysis.















































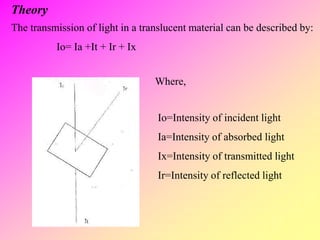
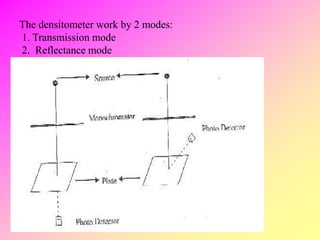

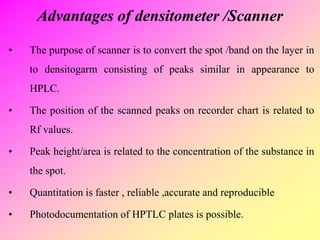



![high performance thin layer chromatography [HPTLC]](https://image.slidesharecdn.com/54-191219130320/85/high-performance-thin-layer-chromatography-HPTLC-63-320.jpg)