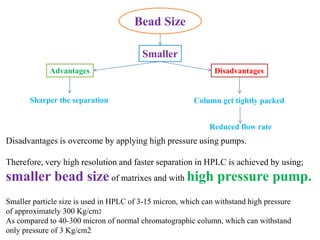
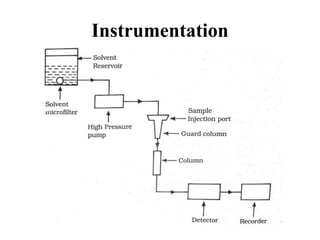
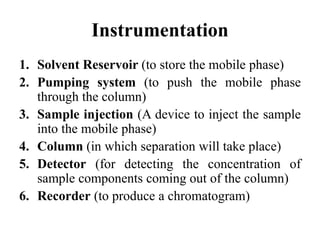
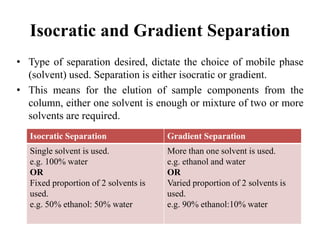
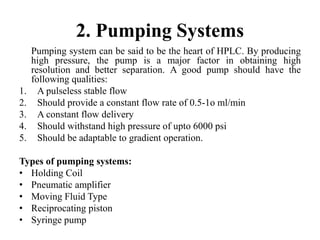
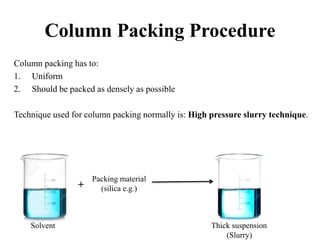
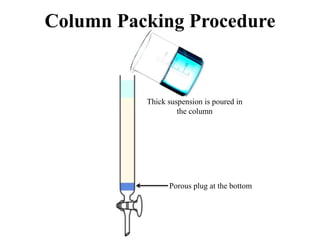
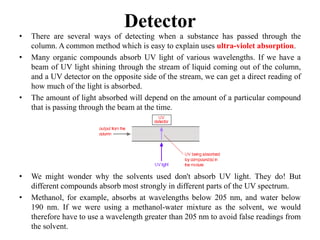
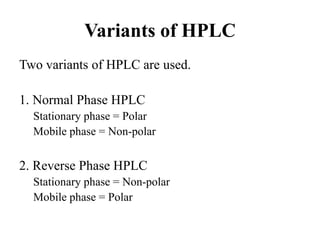
The document discusses High Performance Liquid Chromatography (HPLC). It states that HPLC provides faster separation of compounds compared to other chromatographic techniques due to the use of smaller bead sizes in columns and high pressure pumps. Smaller bead sizes allow for sharper separation but reduce flow rates, which is overcome by applying high pressure. Therefore, HPLC achieves very high resolution and faster separation using smaller bead sizes and high pressure pumps.