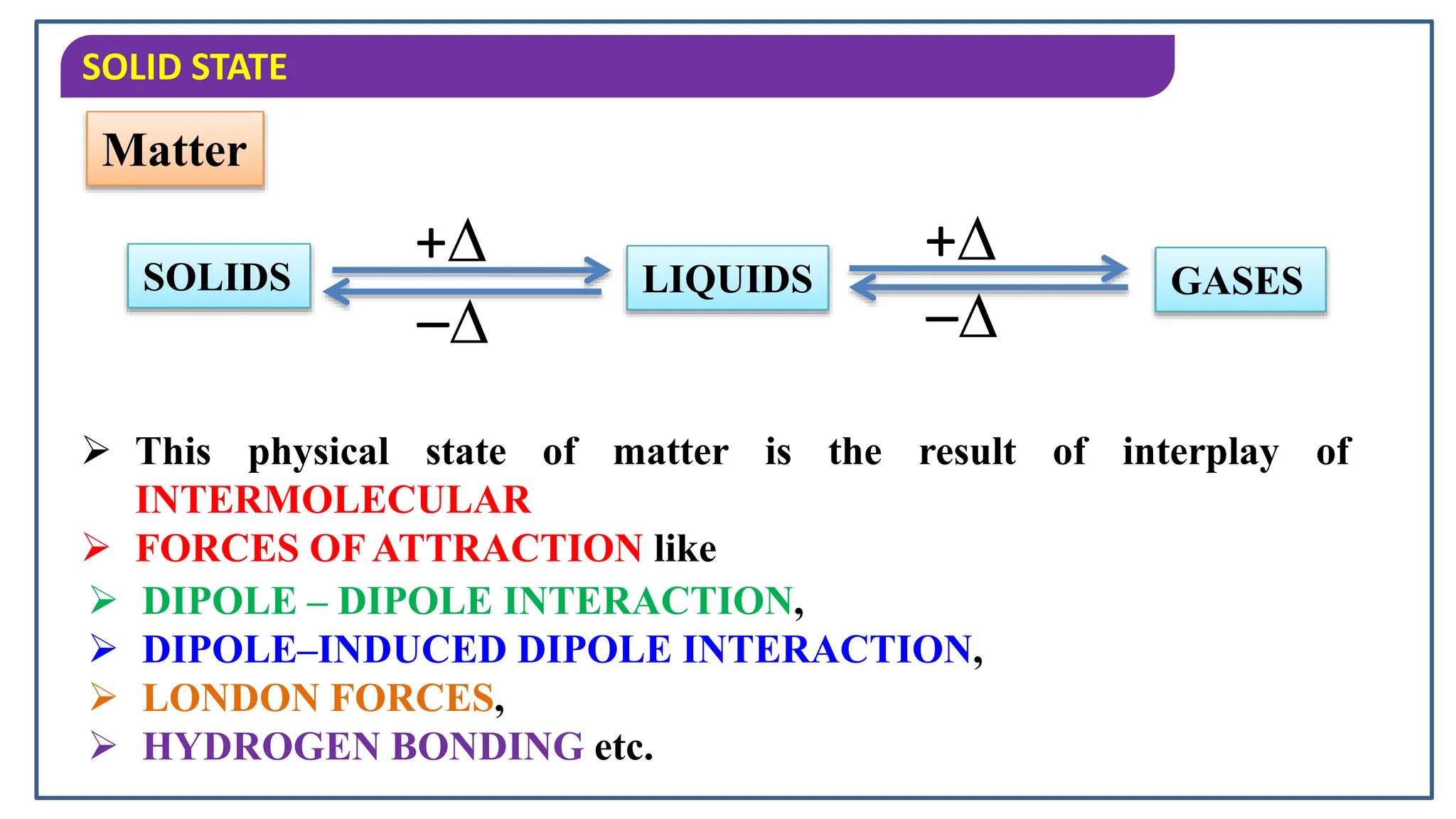
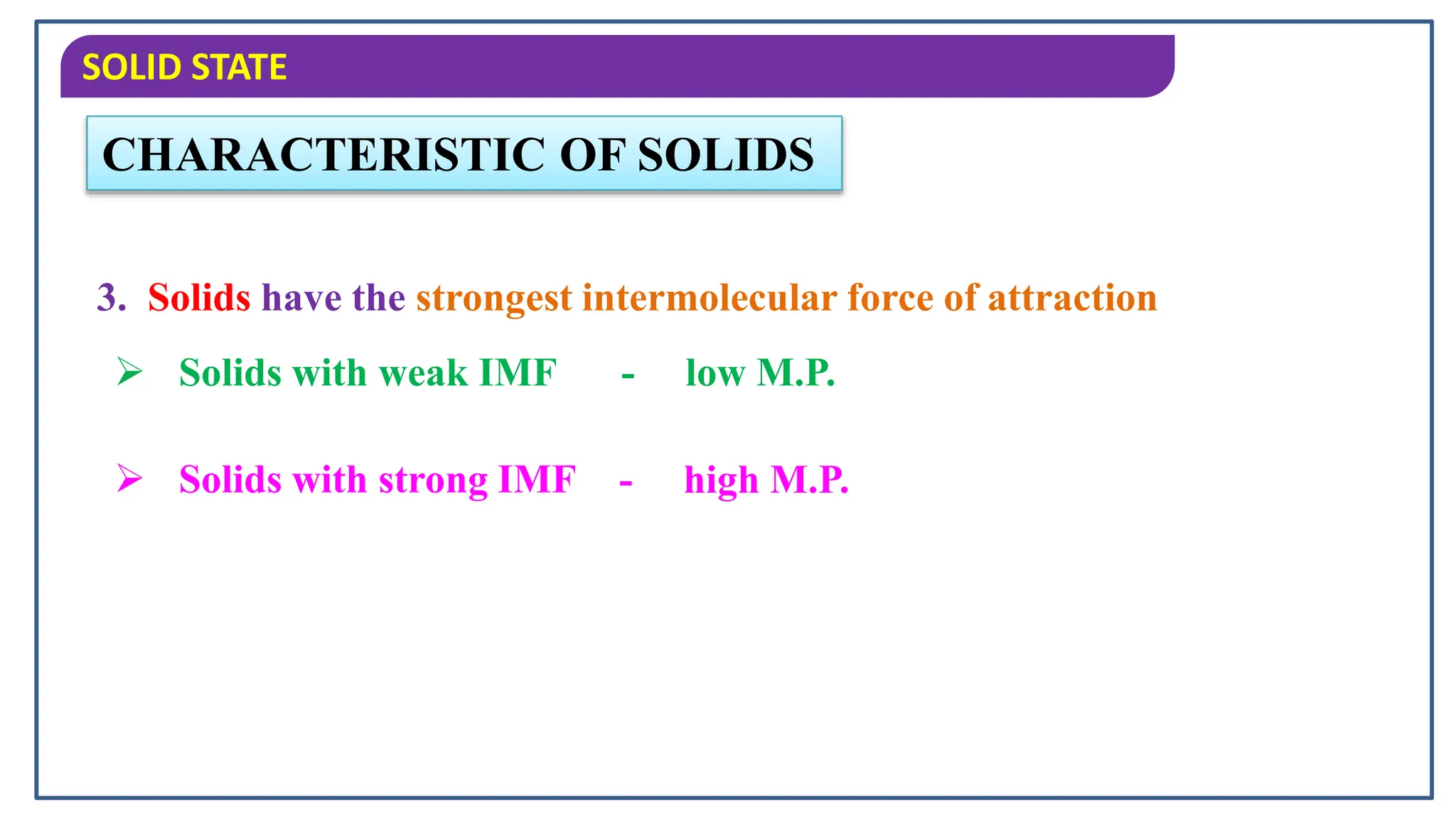
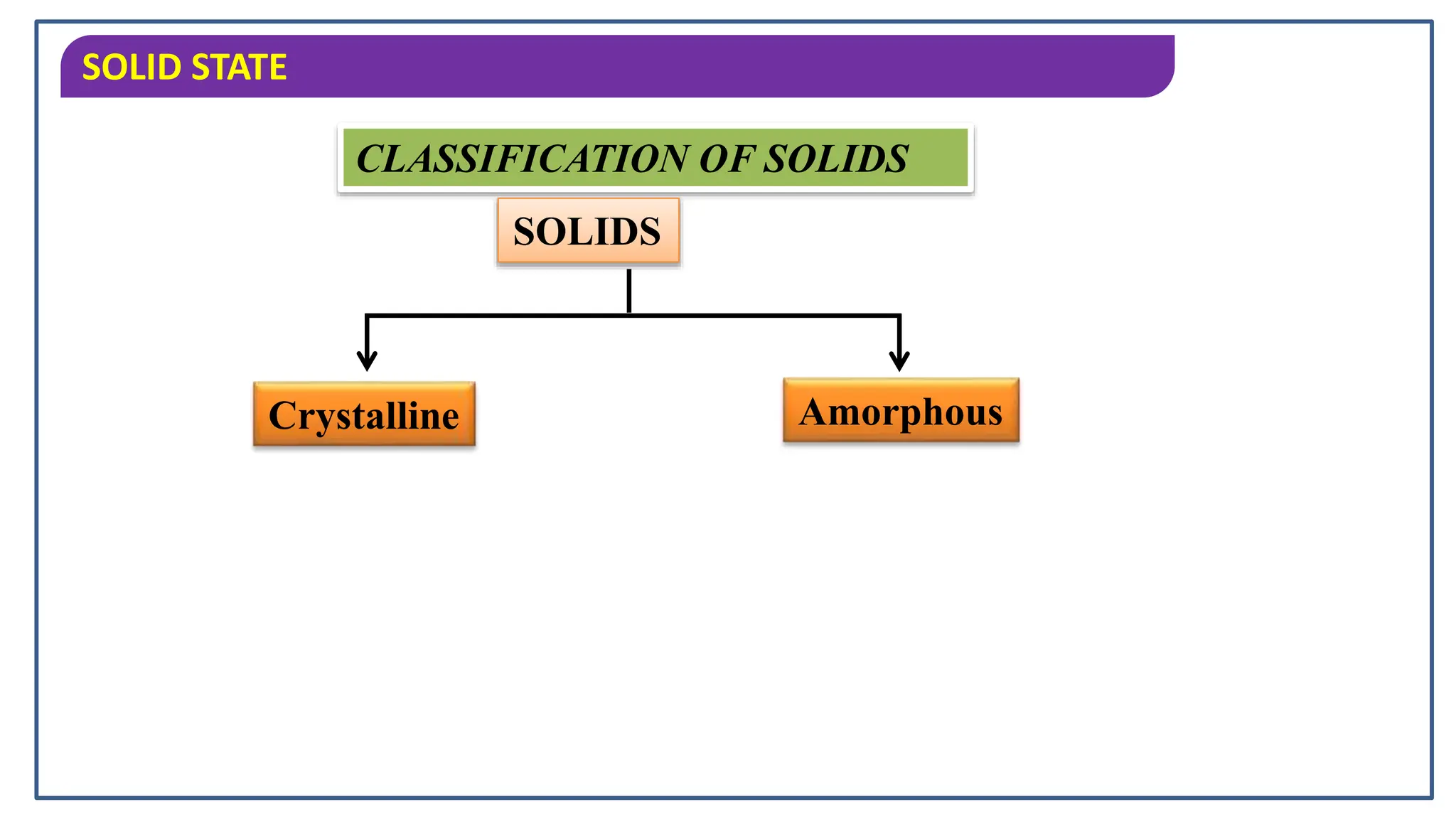
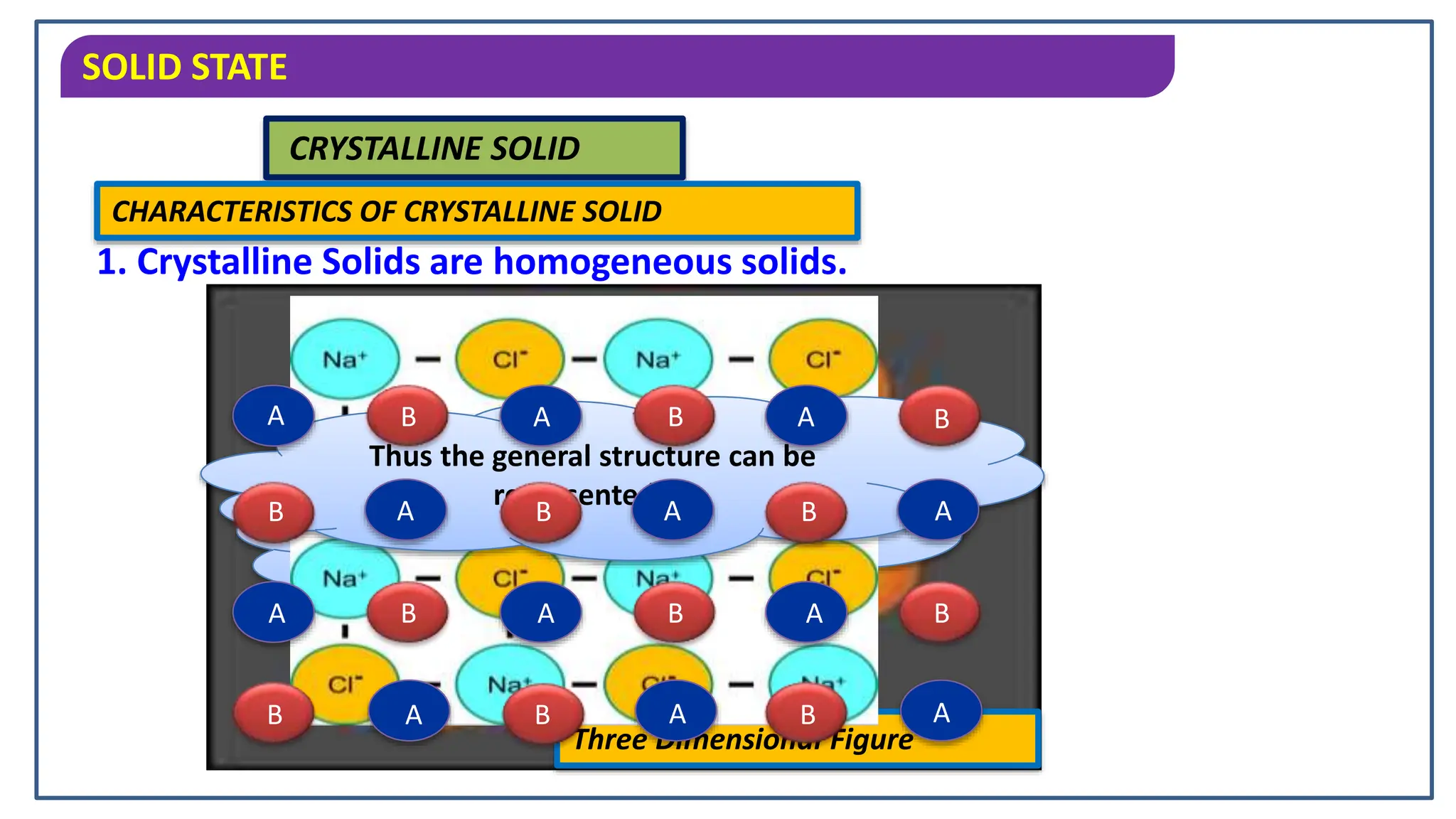
The document discusses the states of matter, specifically focusing on solids, their characteristics and classifications. It explains that solids have fixed mass, volume, shape, and density, resulting from strong intermolecular forces, and details the differences between crystalline and amorphous solids. Key points include the arrangement of particles in solids, their incompressibility, and the properties of crystalline solids such as long-range order.