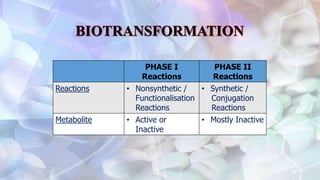
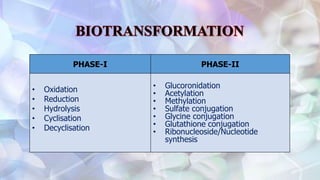
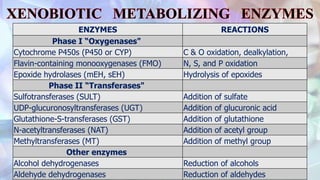
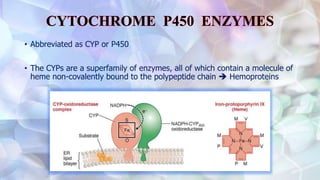
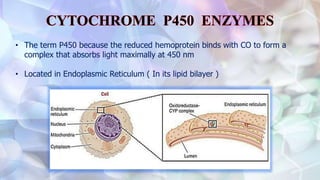
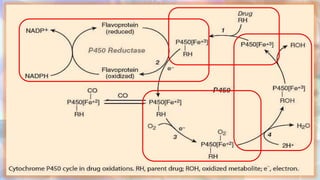
This document discusses drug metabolism and the clinical relevance of biotransformation. It begins by defining biotransformation as the biochemical reactions that alter drugs within the body, and discusses the roles of microsomal enzymes like cytochrome P450 enzymes and UDP glucuronosyl transferases. It then covers the phases of drug metabolism and the enzymes involved in each phase. Specific cytochrome P450 isoforms and their drug substrates are discussed. The effects of enzyme induction and inhibition on drug metabolism and potential drug interactions are summarized. Factors influencing individual differences in drug metabolism like genetic polymorphisms and environmental factors are briefly mentioned in the conclusion.







































![Genetic Polymorphism –
• Based on Metabolic Ratio, individuals are divided into –
1) Poor metabolisers (PM)
2) Extensive metabolisers (EM)
3) Ultra rapid metabolisers (UM)
• [Metabolic Ratio: defined as percent of dose excreted as unchanged drug
divided by the percent of dose excreted as metabolite in urine collected
over a time period after oral ingestion of drug]](https://image.slidesharecdn.com/seminarhepaticmicrisomalenzymesystem-161006065101/85/Hepatic-Micrisomal-Enzyme-System-58-320.jpg)















