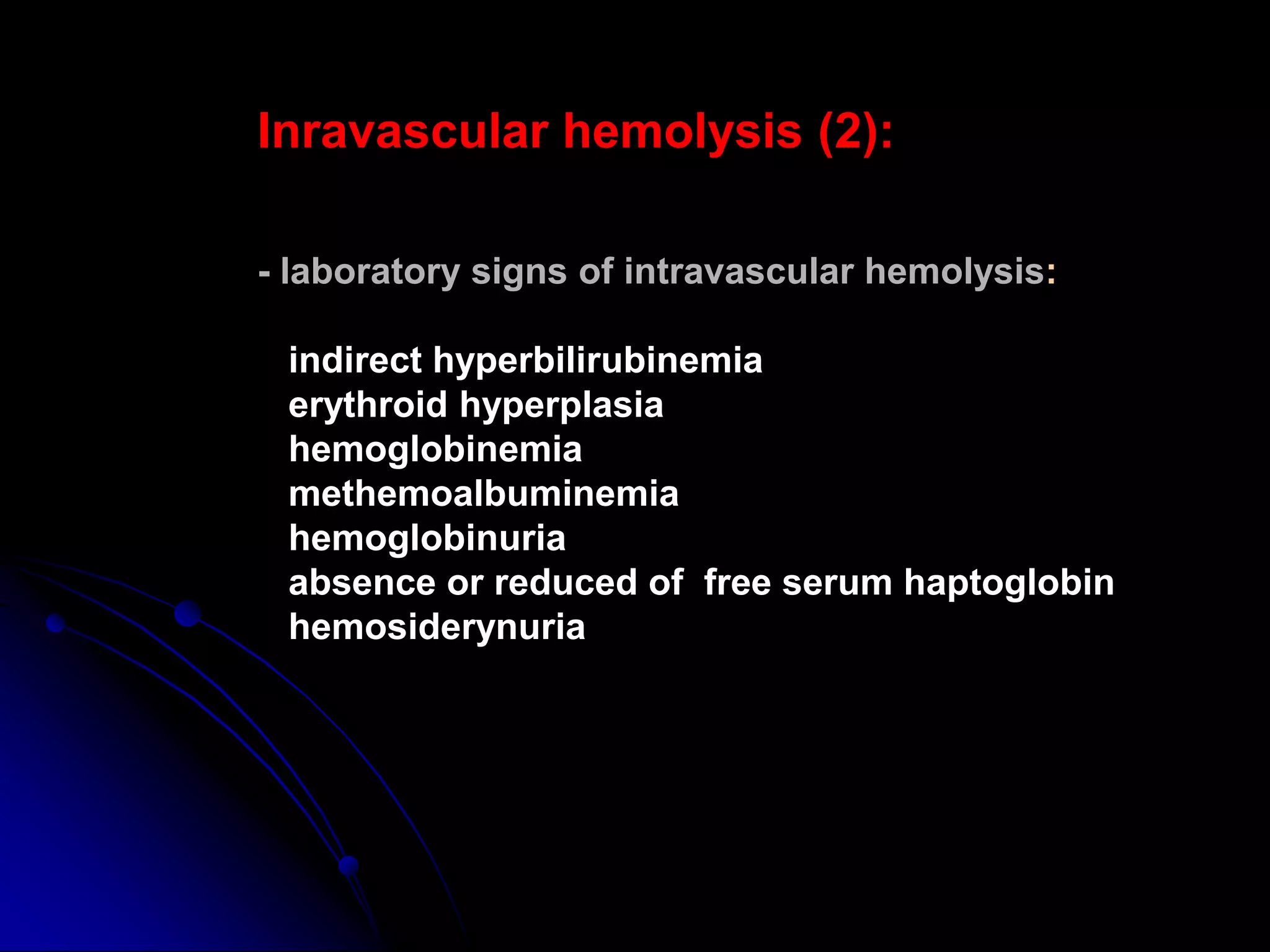
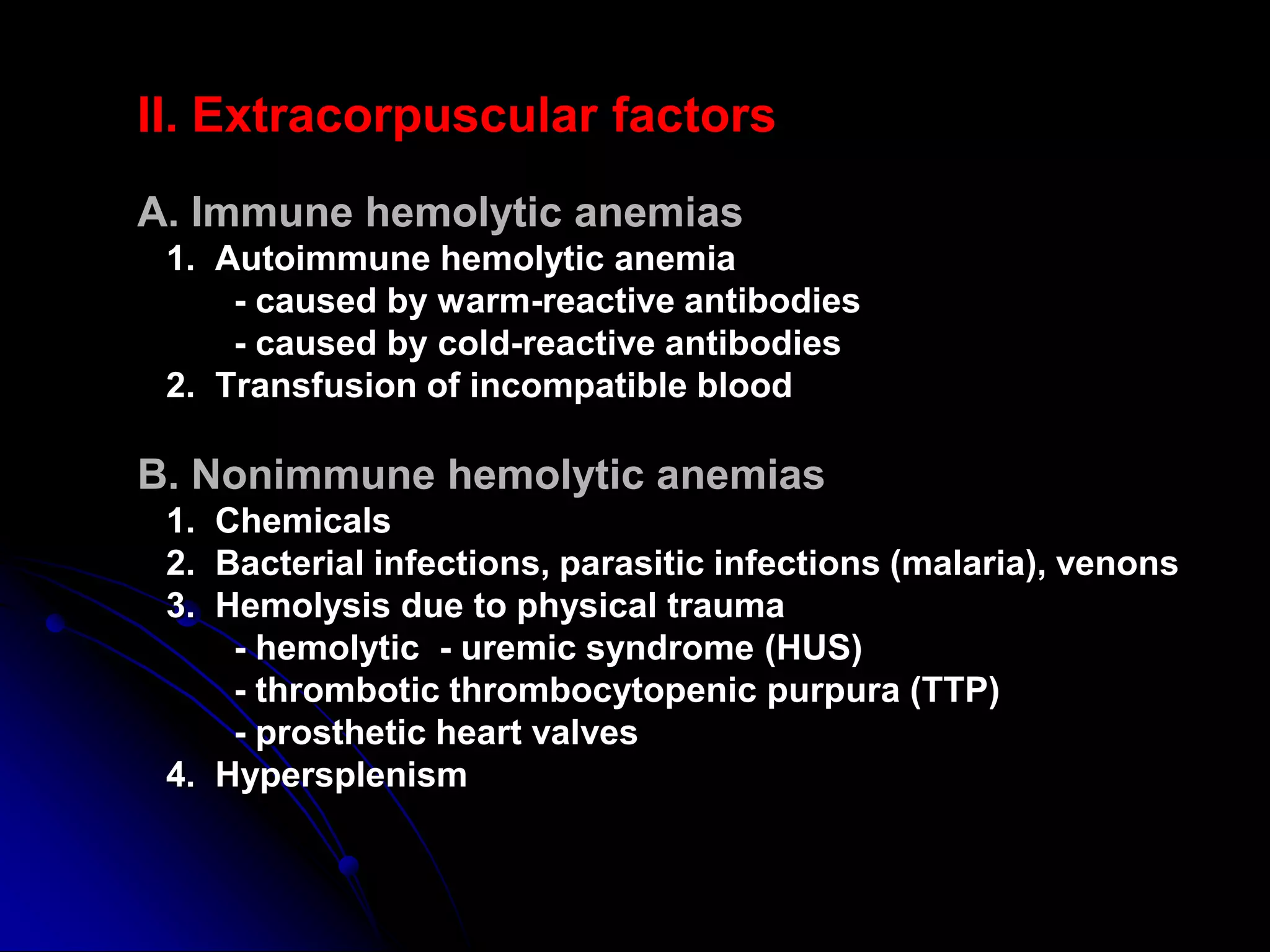
Hemolytic anemias result from increased red blood cell destruction. The document discusses various causes of hemolytic anemia including congenital/hereditary factors like red blood cell membrane defects and enzymatic deficiencies, as well as acquired causes such as autoimmune hemolytic anemia, infection, mechanical trauma, and paroxysmal nocturnal hemoglobinuria. Key signs of hemolytic anemia include pallor, jaundice, splenomegaly, and laboratory findings indicating increased red blood cell breakdown. Management depends on the underlying cause but may involve treatments like blood transfusions, immunosuppressants, or splenectomy.