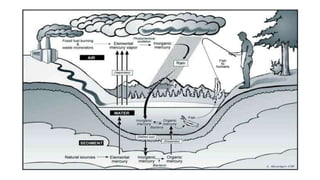
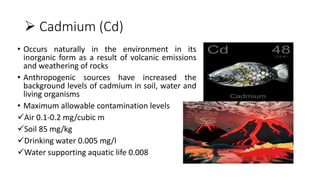
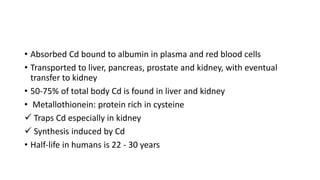
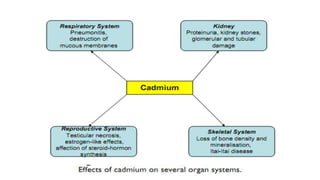
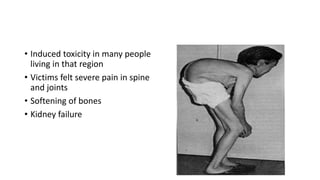
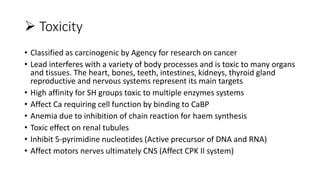
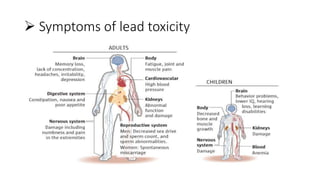
This document discusses toxic minerals including lead, arsenic, mercury, and cadmium. It describes the sources of exposure, absorption, distribution, toxicity effects, and treatment options for poisoning from each of these heavy metals. Lead is discussed in most depth, outlining its various non-occupational exposure sources and noting the EPA limits for levels in drinking water, soil, and air. Symptoms of lead toxicity and chelation therapy using dimercaprol are also summarized.














![ Treatment of lead Toxicity
• Dimercaprol (also referred to as British antilewisite [BAL])
• Chelating agent Removes intra cellular and extracellular lead
• Recommended as an agent of first choice for patients with lead
toxicity.
• With high BLLs (i.e, > 70 µg/dl), it is used in conjunction with EDTA
• Allows extracellular lead to be eliminated through renal system](https://image.slidesharecdn.com/toxicminerals-161127152324/85/Toxic-minerals-17-320.jpg)