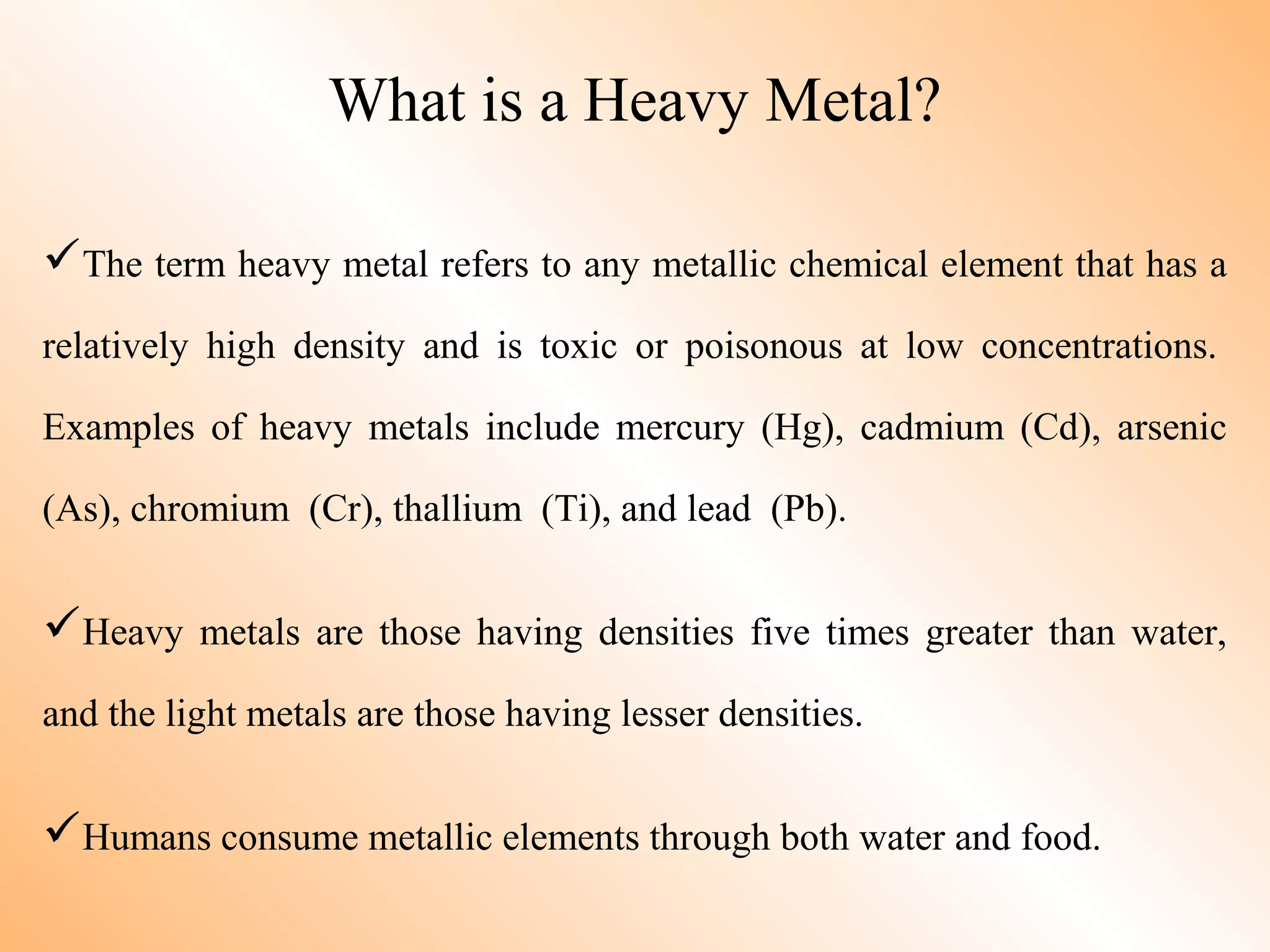
Heavy metal pollution in soil is a serious problem. Some key points:
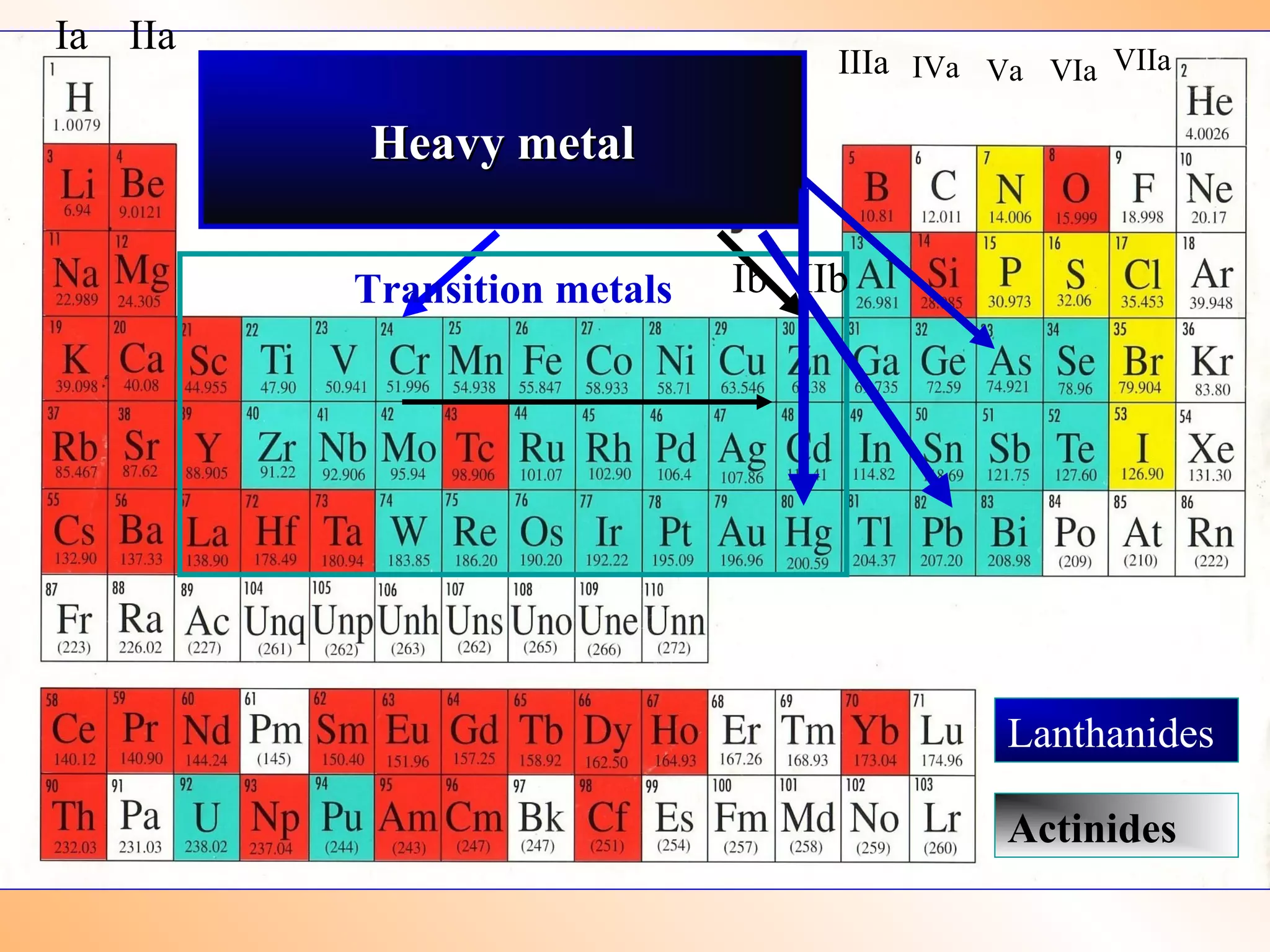
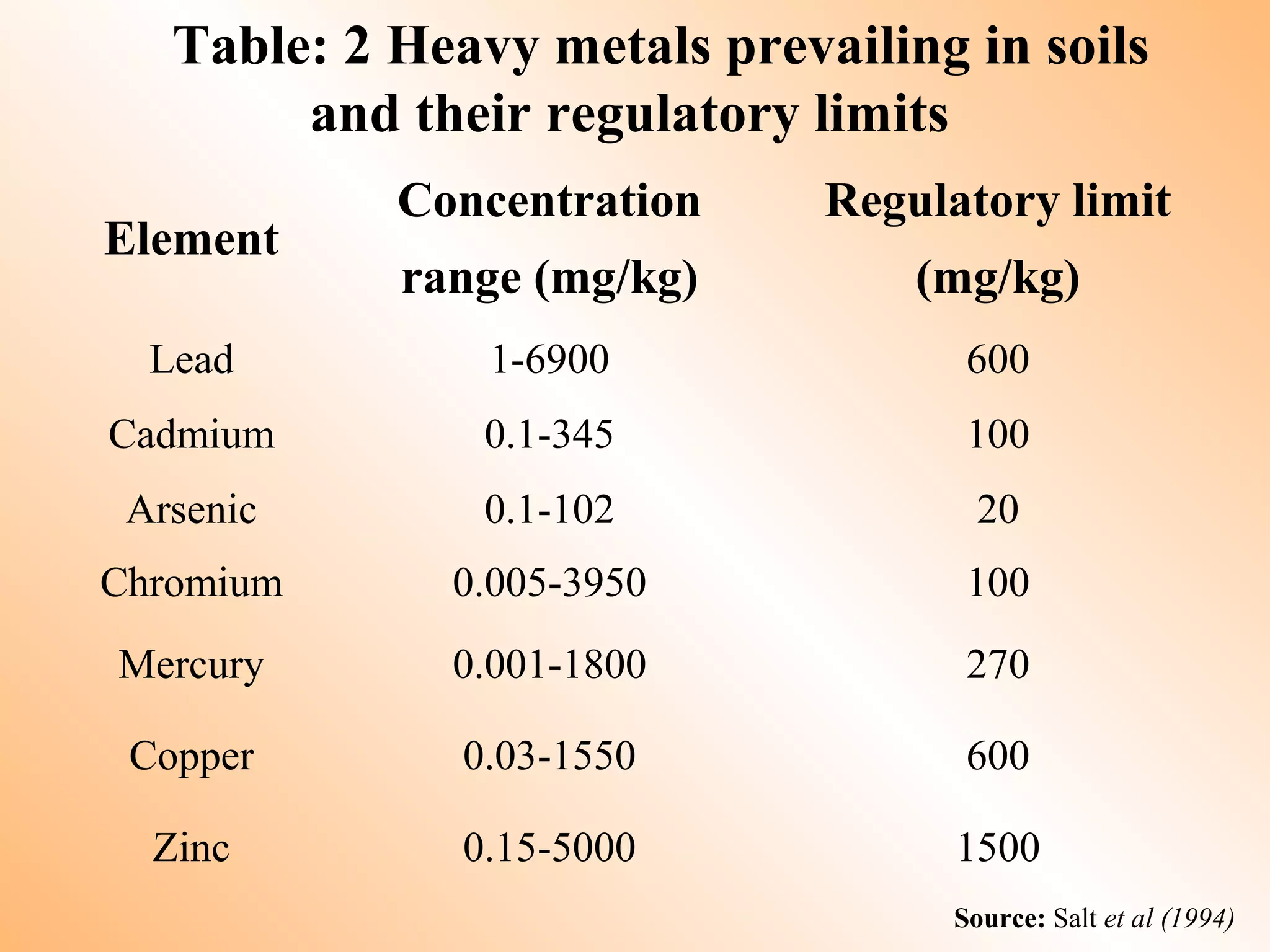
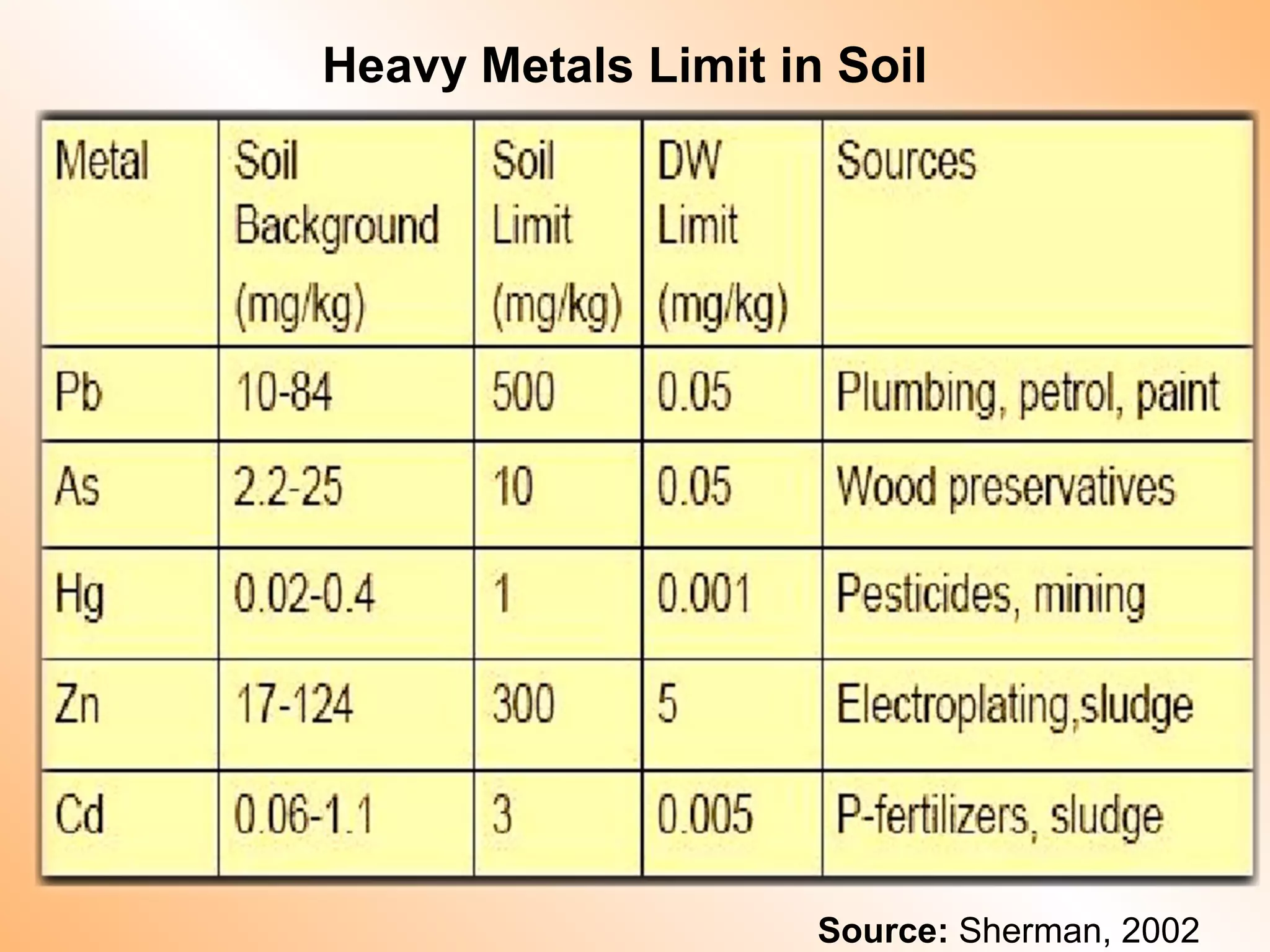
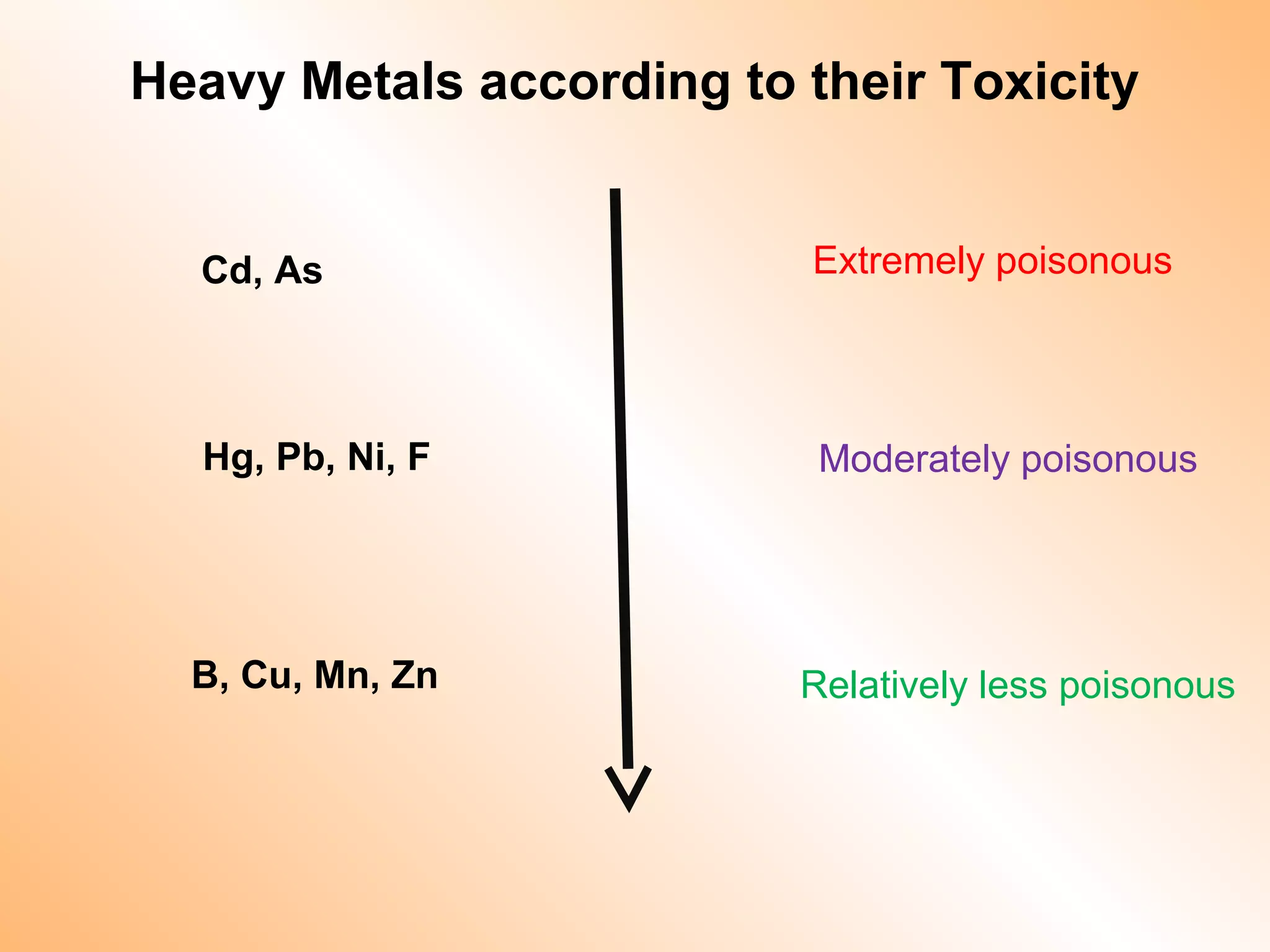
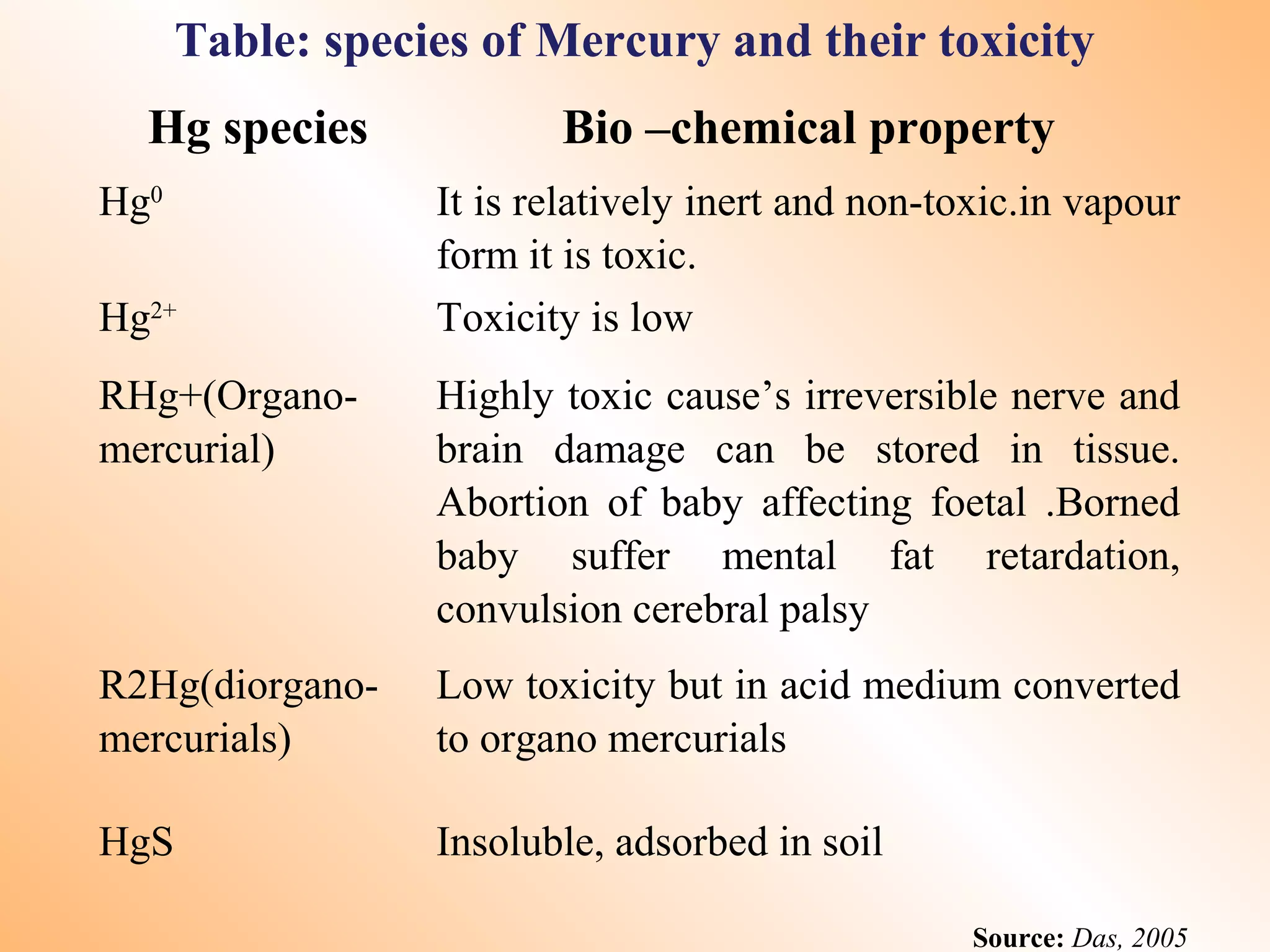
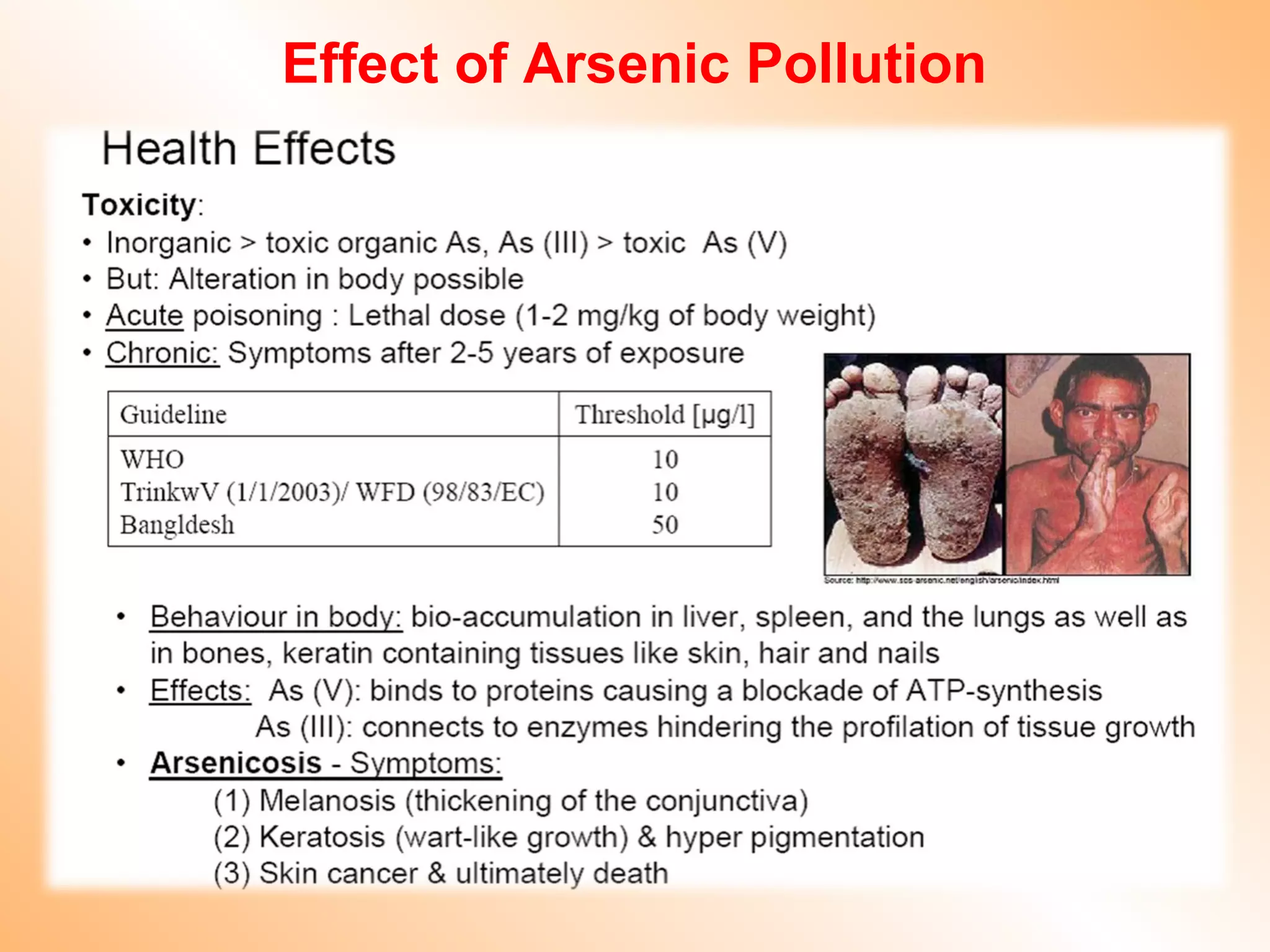
- Heavy metals like lead, cadmium, arsenic, chromium, and mercury are toxic even in small amounts and can accumulate in the food chain.
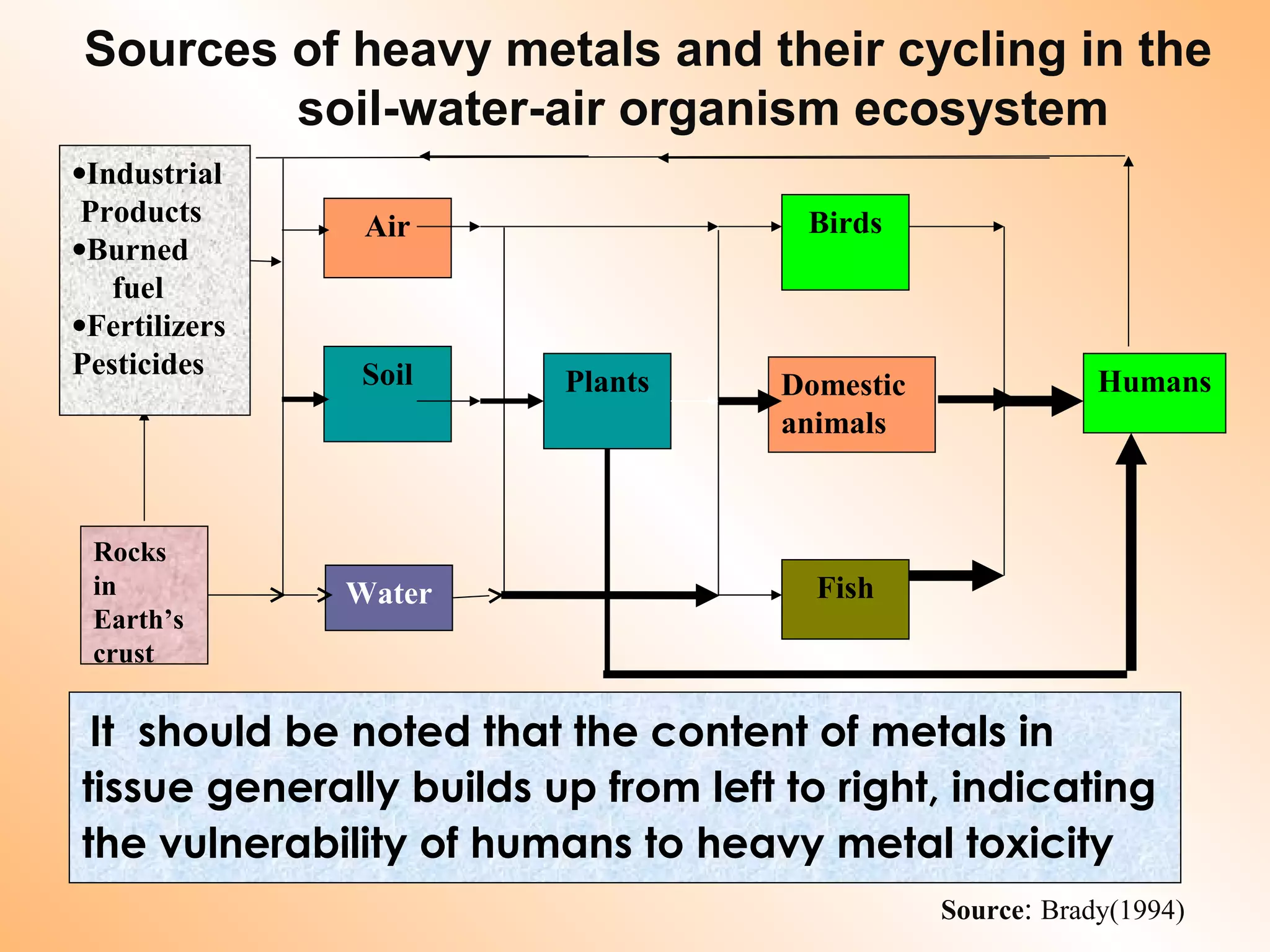
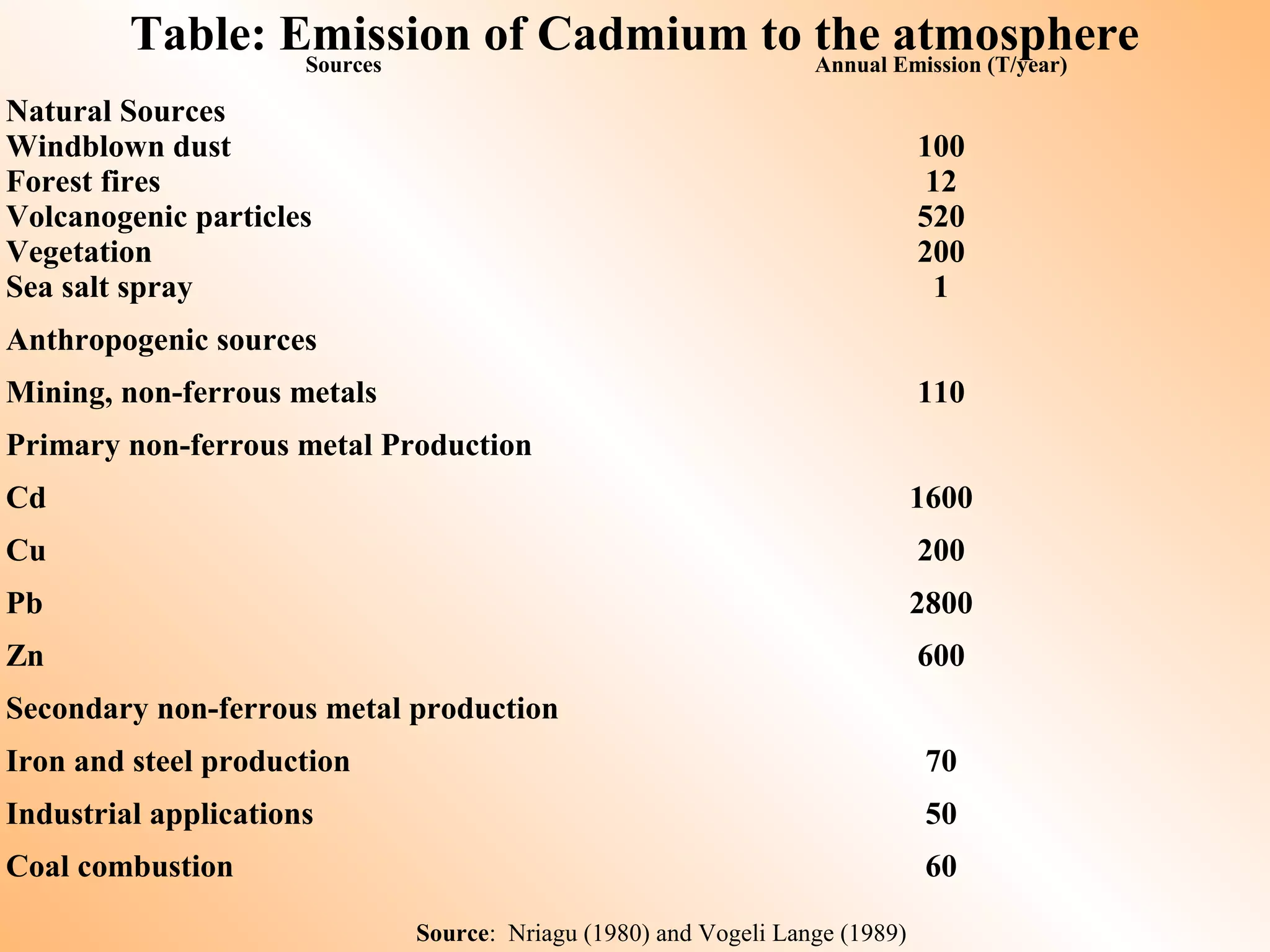
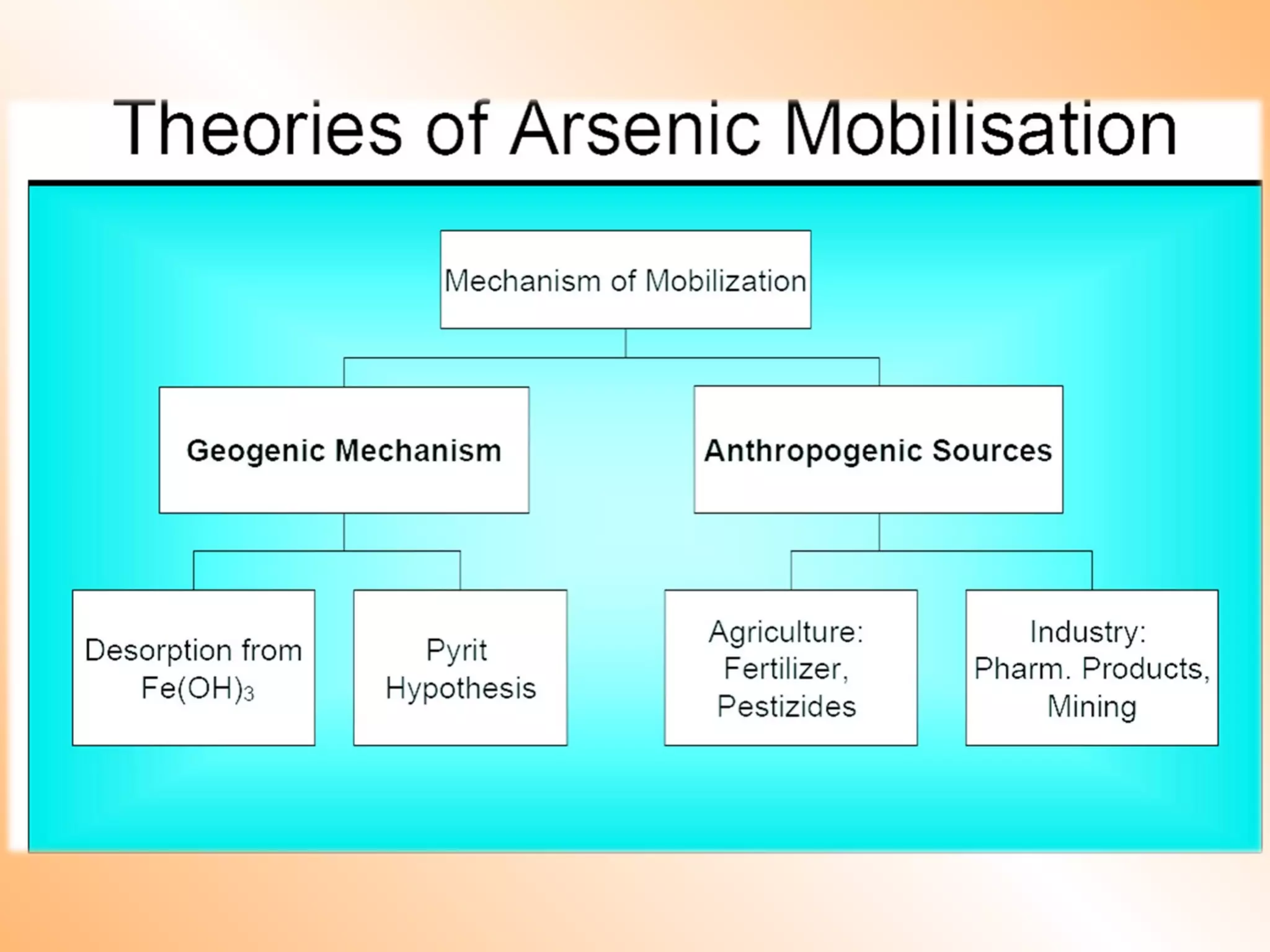
- Sources of heavy metal pollution include industrial, agricultural, and mining activities which release these metals into the environment.
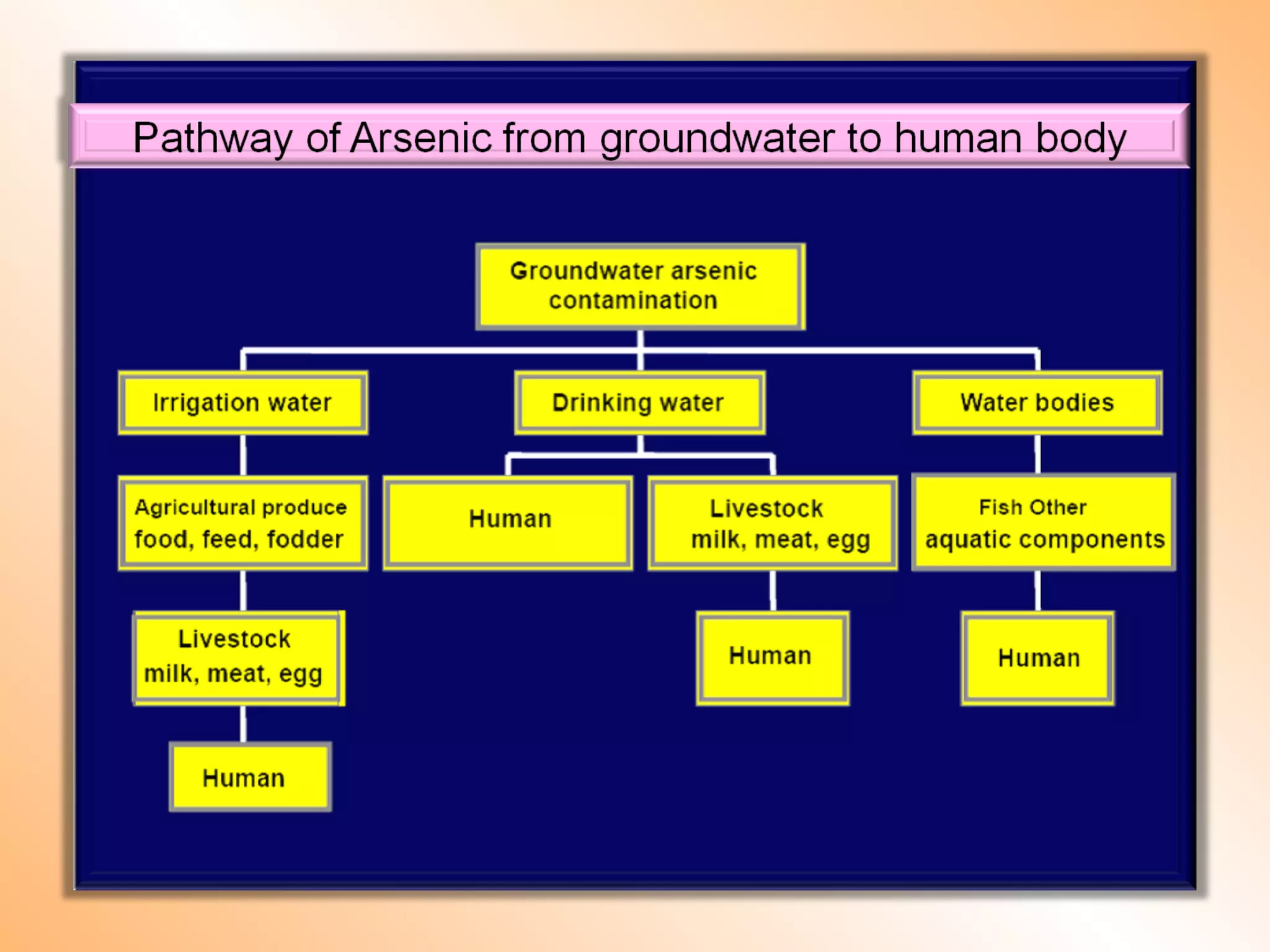
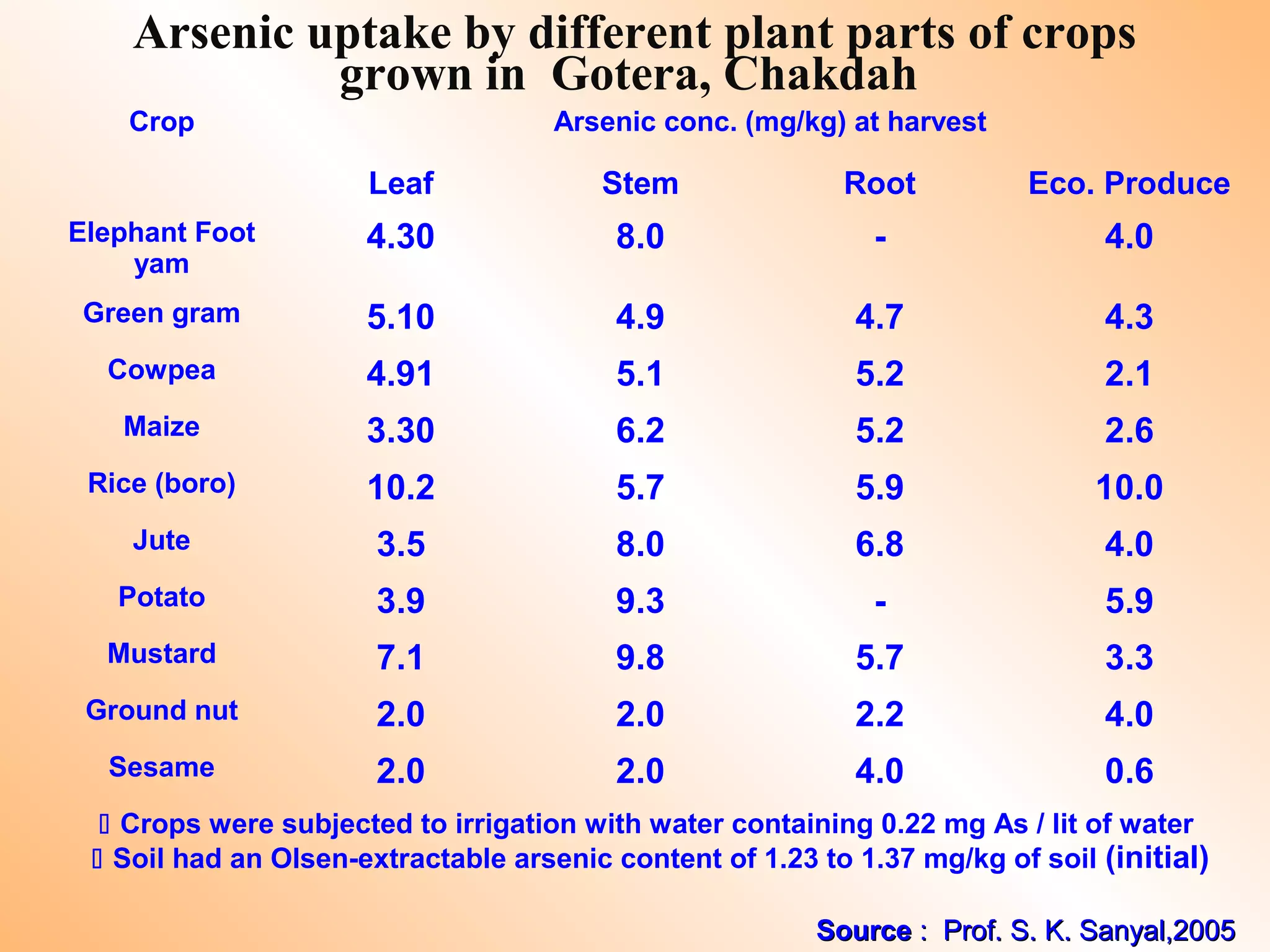
- Heavy metals can be taken up by plants and crops irrigated with contaminated water, accumulating in plant tissues and eventually entering the food chain. This poses risks to human and animal health.
- Remediating contaminated soils requires understanding the chemical processes by which heavy metals move and change form in the soil-water-air system over time. Mitigation strategies aim to reduce





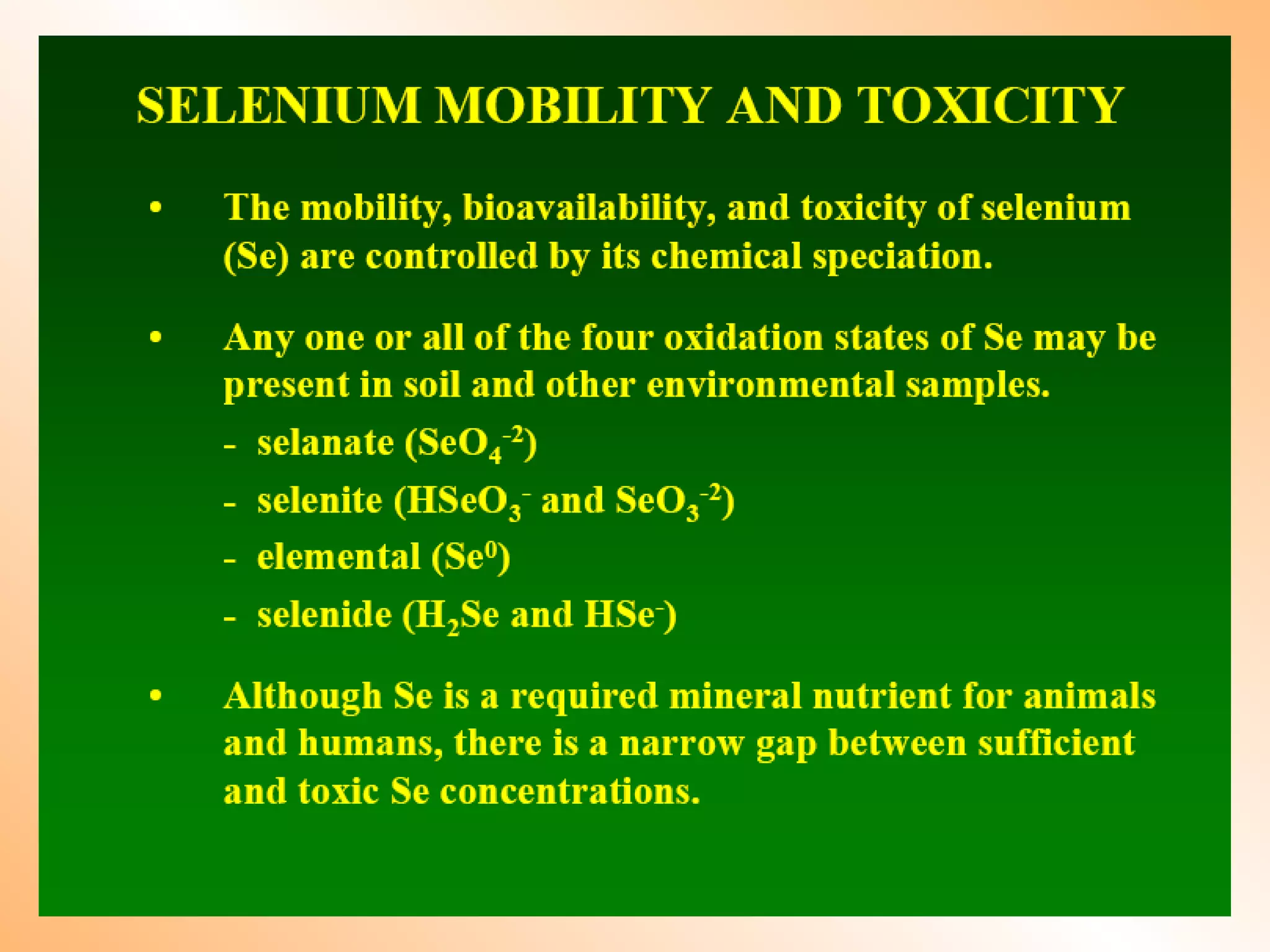

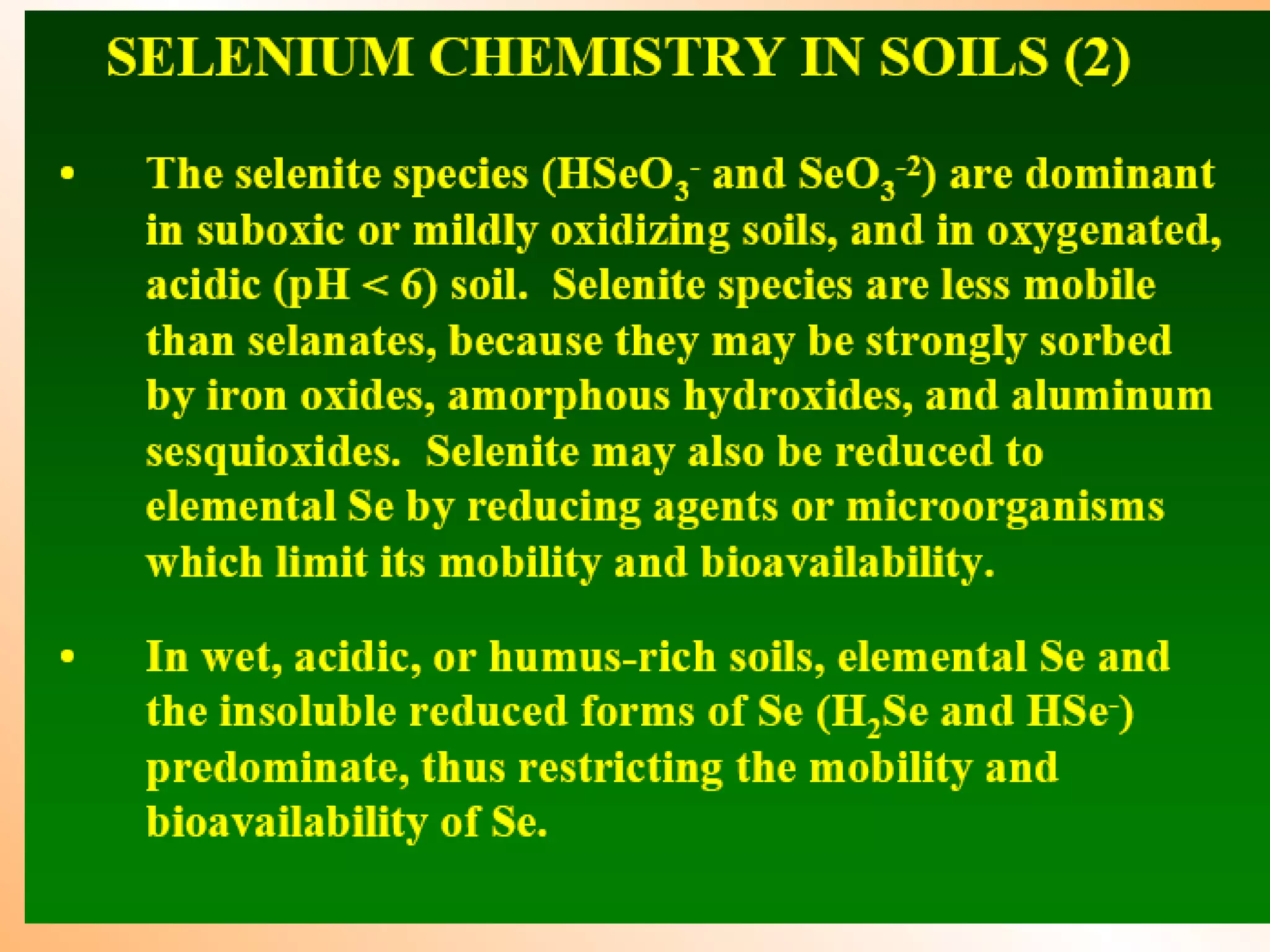
![Fate of selenite in soil (1:10 water extract)
Heavy metal load field experiment: Dr. Imre Kádár, Nagyhörcsök
0
100000
200000
300000
400000
500000
600000
700000
800000
900000
1000000
0 10 20
duration [min]
ICP-MSsignal[CPS]
0
5000
10000
15000
20000
25000
0 10 20
duration [min]
ICP-MSsignal[CPS]
0
5000
10000
15000
20000
25000
0 10 20
duration [min]
ICP-MSsignal[CPS]
1993 1993 2003
oxidation
selenite
selenate
organic selenium
Conclusion: selenite will be oxidised in soil to selenate
selenate more available for plants (it is analogous to sulphate)
risk of leaching
selenite
selenate](https://image.slidesharecdn.com/heavymetalpollution-140314130110-phpapp01/75/Heavy-metal-pollution-in-soil-and-its-mitigation-aspect-by-Dr-Tarik-Mitran-17-2048.jpg)







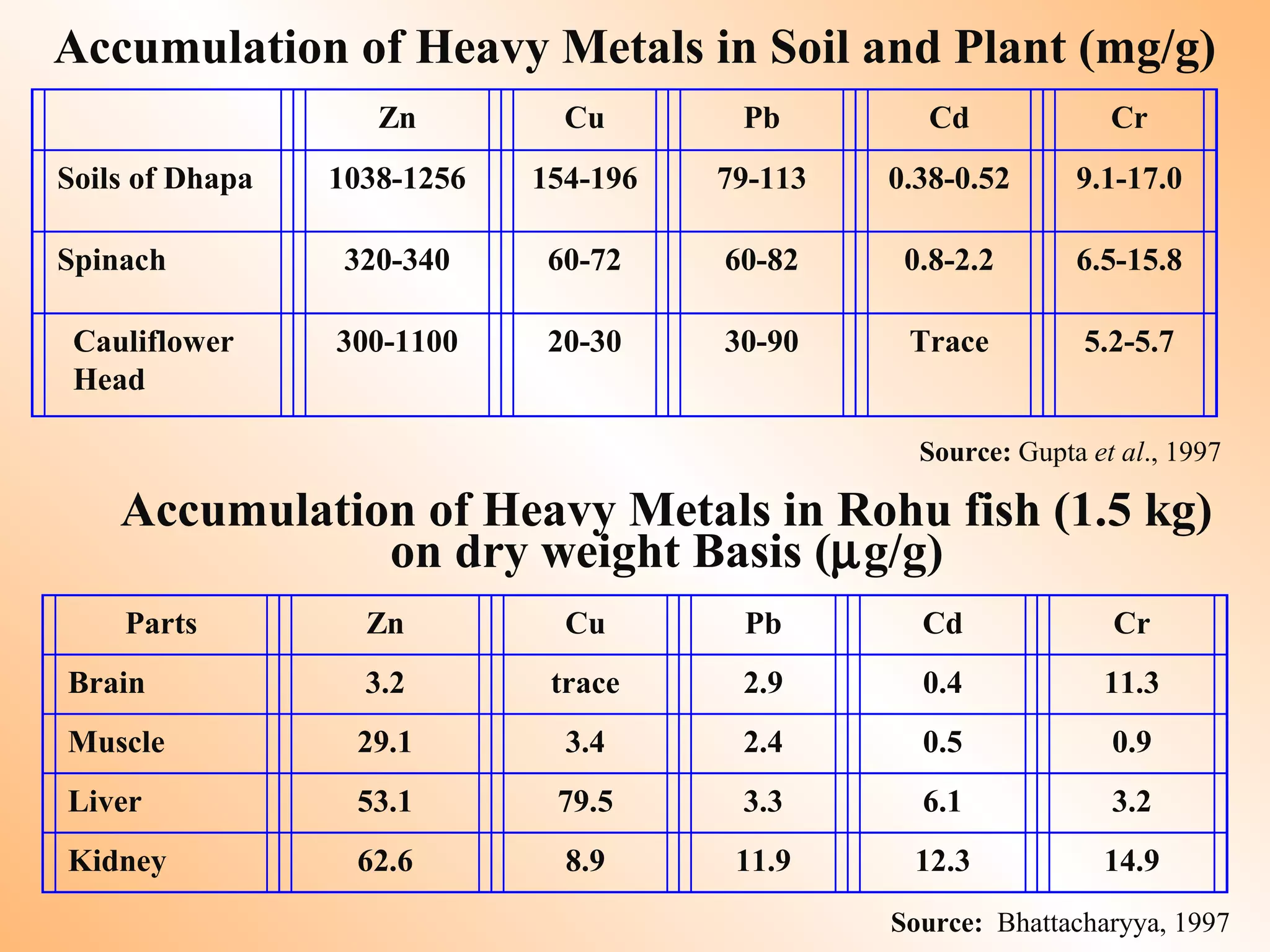
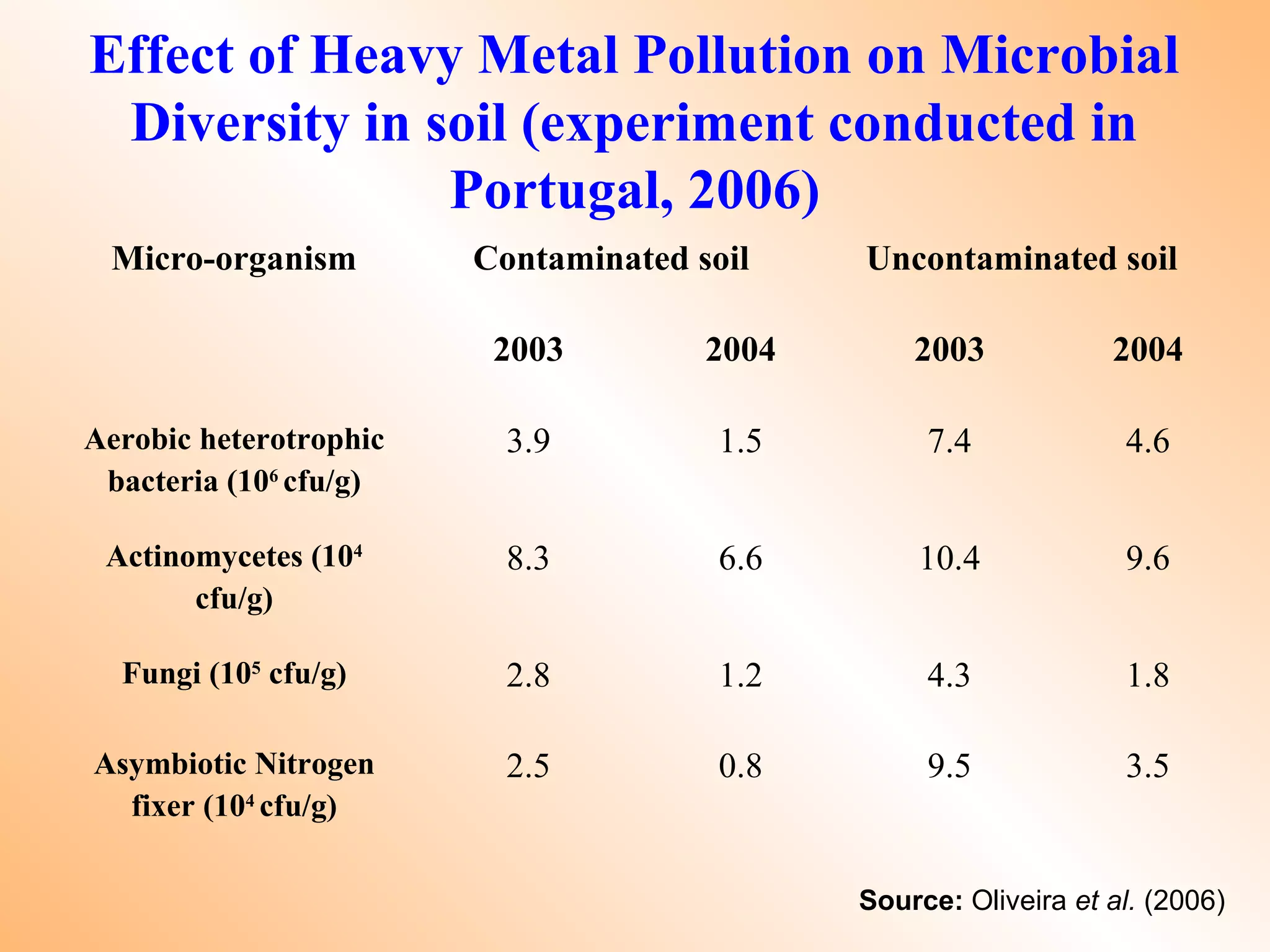
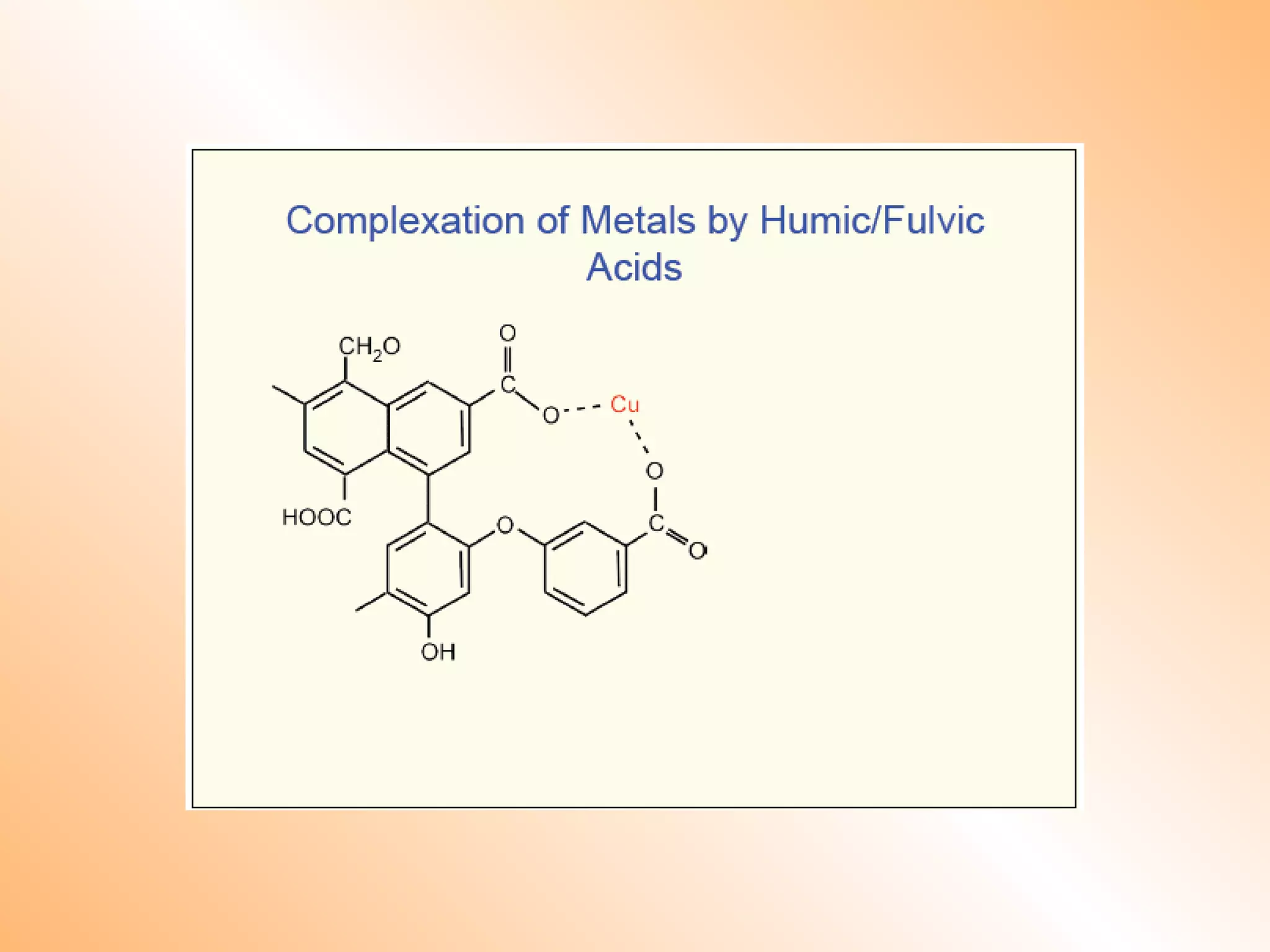

![Remedial / Mitigation Options
1. Optimum conjunctive use of ground & surface water
[ e.g. harvested rainwater]
2. Irrigation with pond-stored groundwater – decontamination facilitated
by rainfall and sedimentation
3. Recharge groundwater resource
4. Enhance water use efficiency (optimum water management)
5. Prefer low-water requiring farmer-attractive cropping sequences
(especially for the lean period)
6. Increased use of FYM and other manures + green manure crops,
inclusion of pulses/other legumes as well as application of appropriate
amendments (Zn/Fe salts as and where applicable)
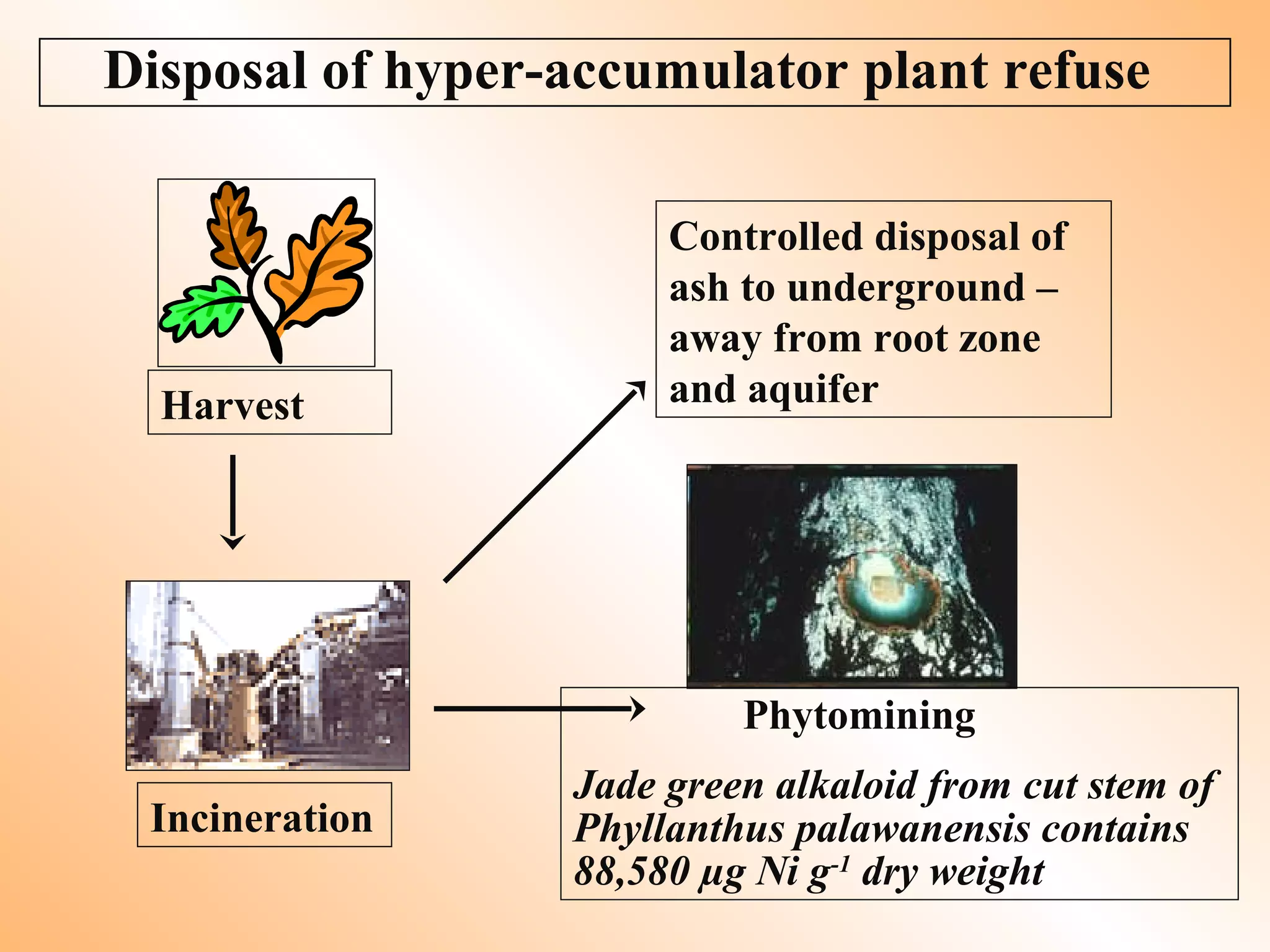
7. Cost-effective phytoremediation options
8. Creation of general awareness: Mass campaigning, holding of farmers’
day, field demonstrations, socioeconomic factors
(Source: Sanyal, 2008)](https://image.slidesharecdn.com/heavymetalpollution-140314130110-phpapp01/75/Heavy-metal-pollution-in-soil-and-its-mitigation-aspect-by-Dr-Tarik-Mitran-51-2048.jpg)
