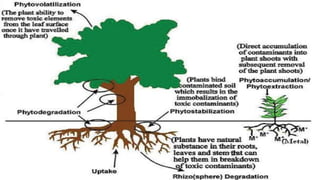
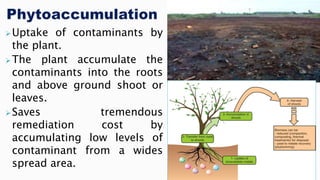
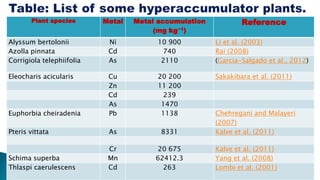
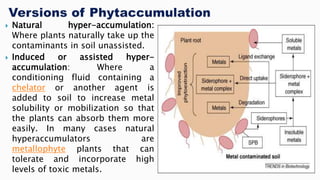
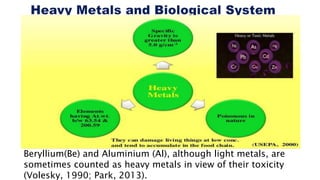
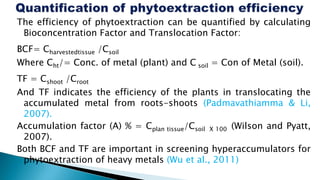
This document provides an overview of phytoremediation and phytoaccumulation. Phytoremediation uses various plants to remove, transfer, stabilize, and destroy contaminants in soil and groundwater. Specifically, phytoaccumulation uses plants or algae to remove contaminants from soils, sediments, or water by taking up contaminants into harvestable plant biomass. Certain plants called hyperaccumulators are especially effective at phytoaccumulation due to their ability to absorb and store heavy metals at concentrations much higher than normal plants. The efficiency of phytoaccumulation can be quantified by calculating bioconcentration factors and translocation factors. While phytoaccumulation takes longer than other remediation methods, it is more cost