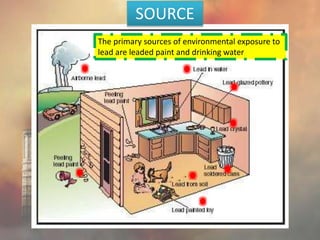
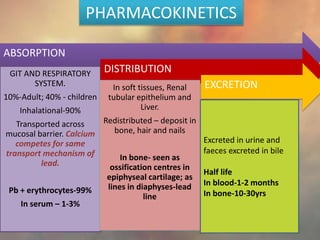
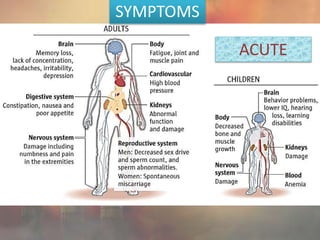
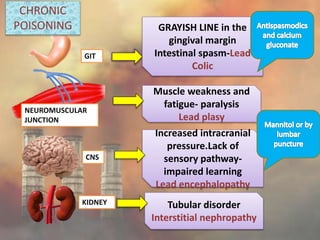
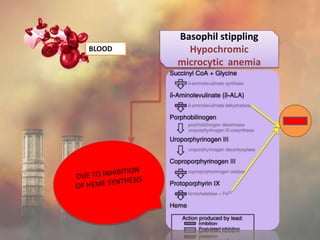
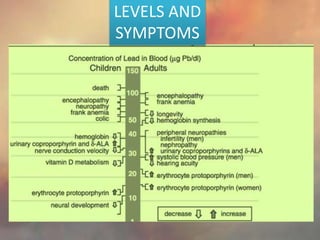
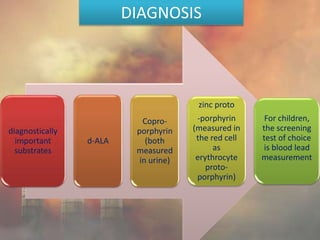
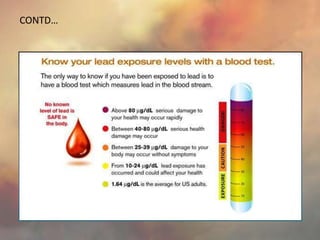
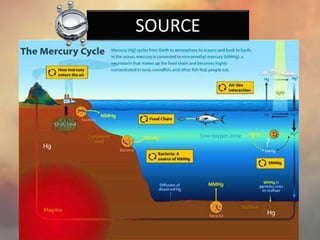
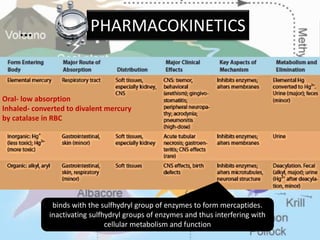
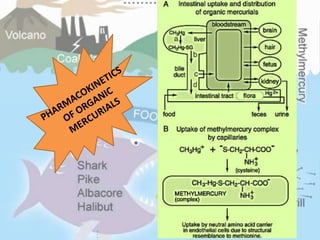
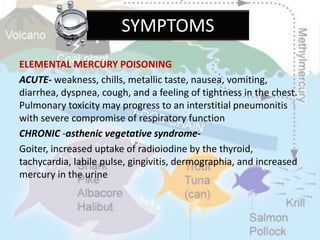
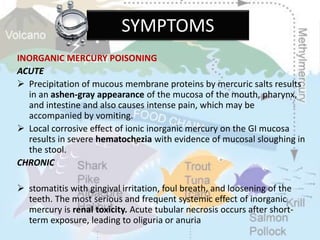
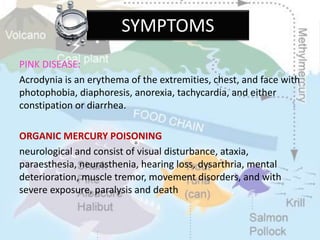
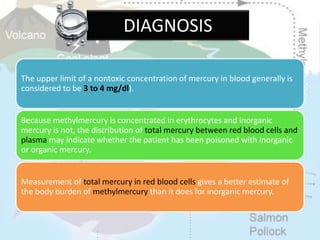
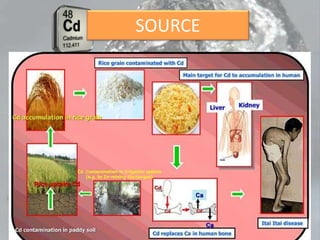
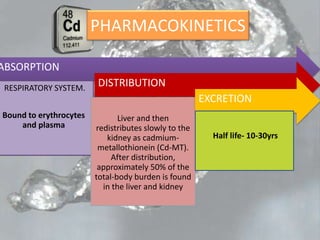
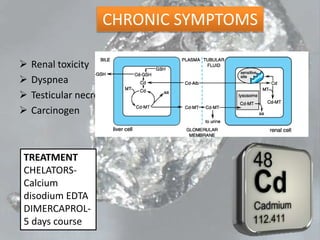
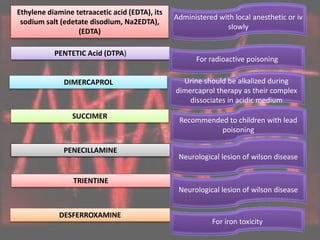
This document discusses heavy metal poisoning from metals such as lead, mercury, cadmium, and others. It covers the sources of exposure, pharmacokinetics, symptoms of acute and chronic poisoning, diagnosis, and treatment methods. Heavy metals are absorbed from the environment through water, food, and industrial exposures. They can cause neurological, gastrointestinal, renal and other organ system effects. Diagnosis involves measuring metal levels in blood and urine. Treatments include chelating agents like EDTA and dimercaprol that bind metals and remove them from the body.