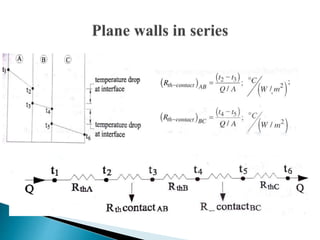
This document discusses heat transfer by conduction. It defines conduction as the transfer of heat through a material by molecular interaction and without bulk motion. Fourier's law of heat conduction states that the rate of heat transfer is proportional to the temperature gradient and the area. The document presents the equations for one-dimensional heat conduction through a plane wall and a composite wall made of different materials. It also lists the assumptions of Fourier's law, such as steady-state conditions and homogeneous/isotropic materials.