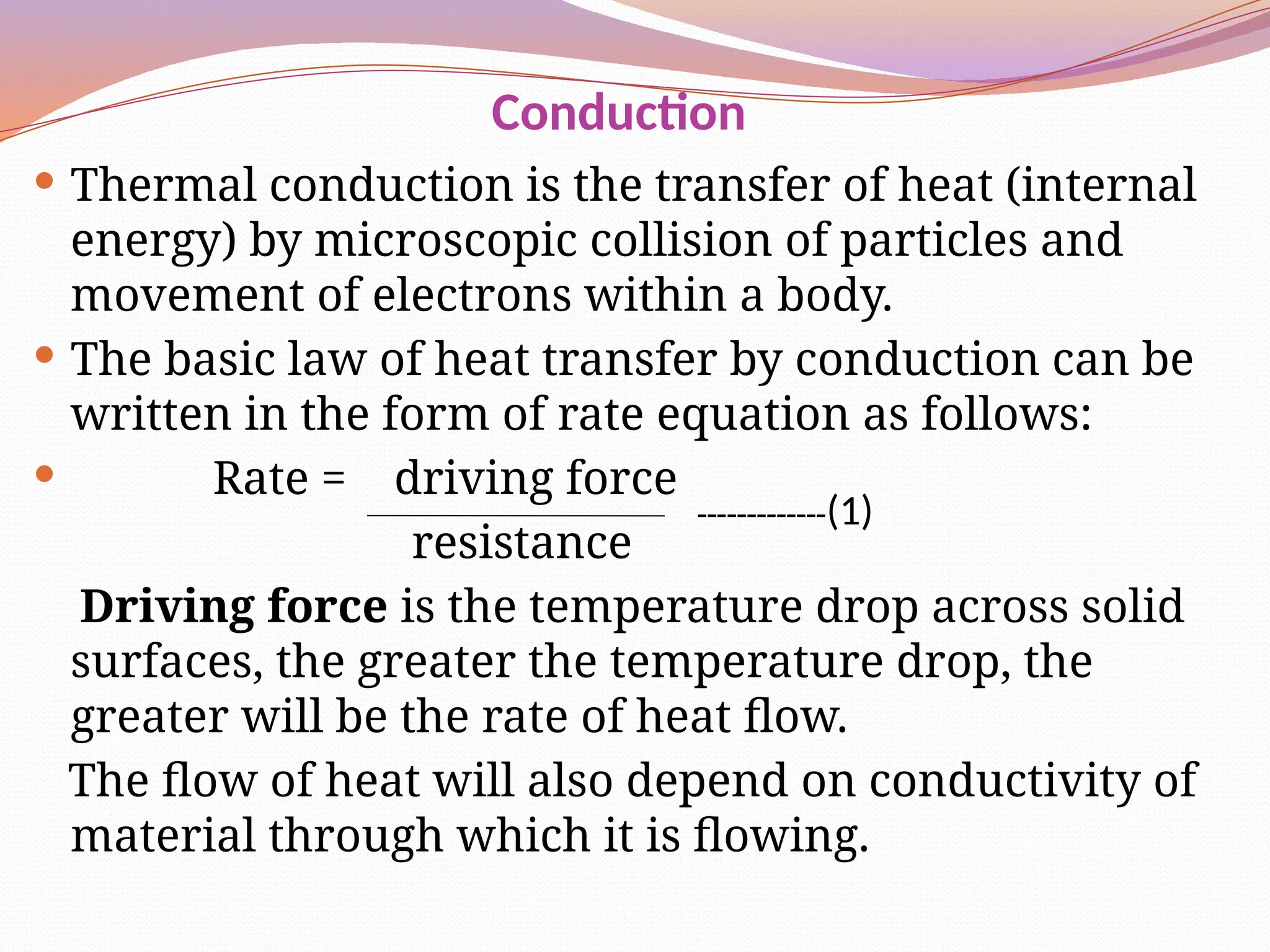
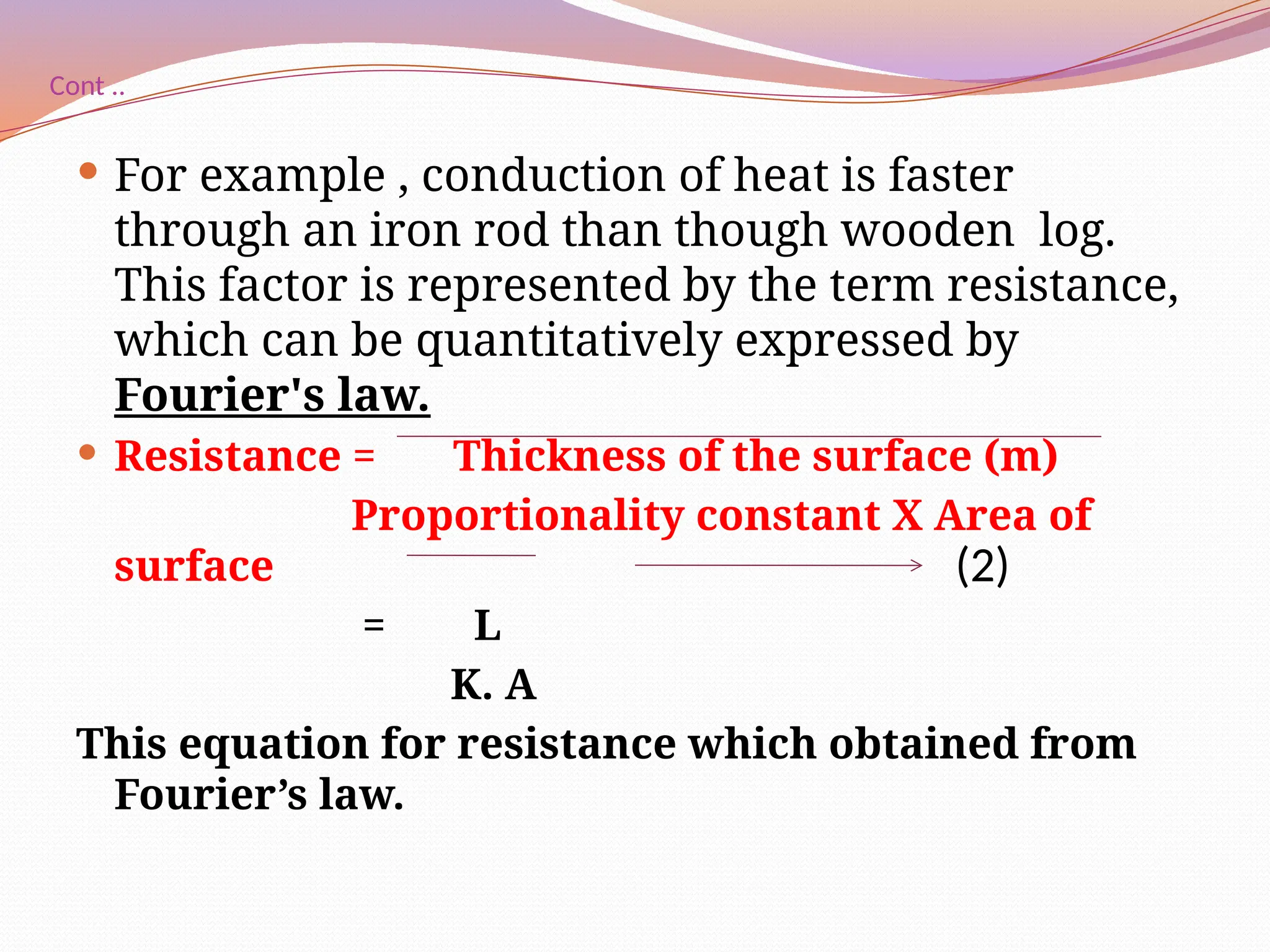
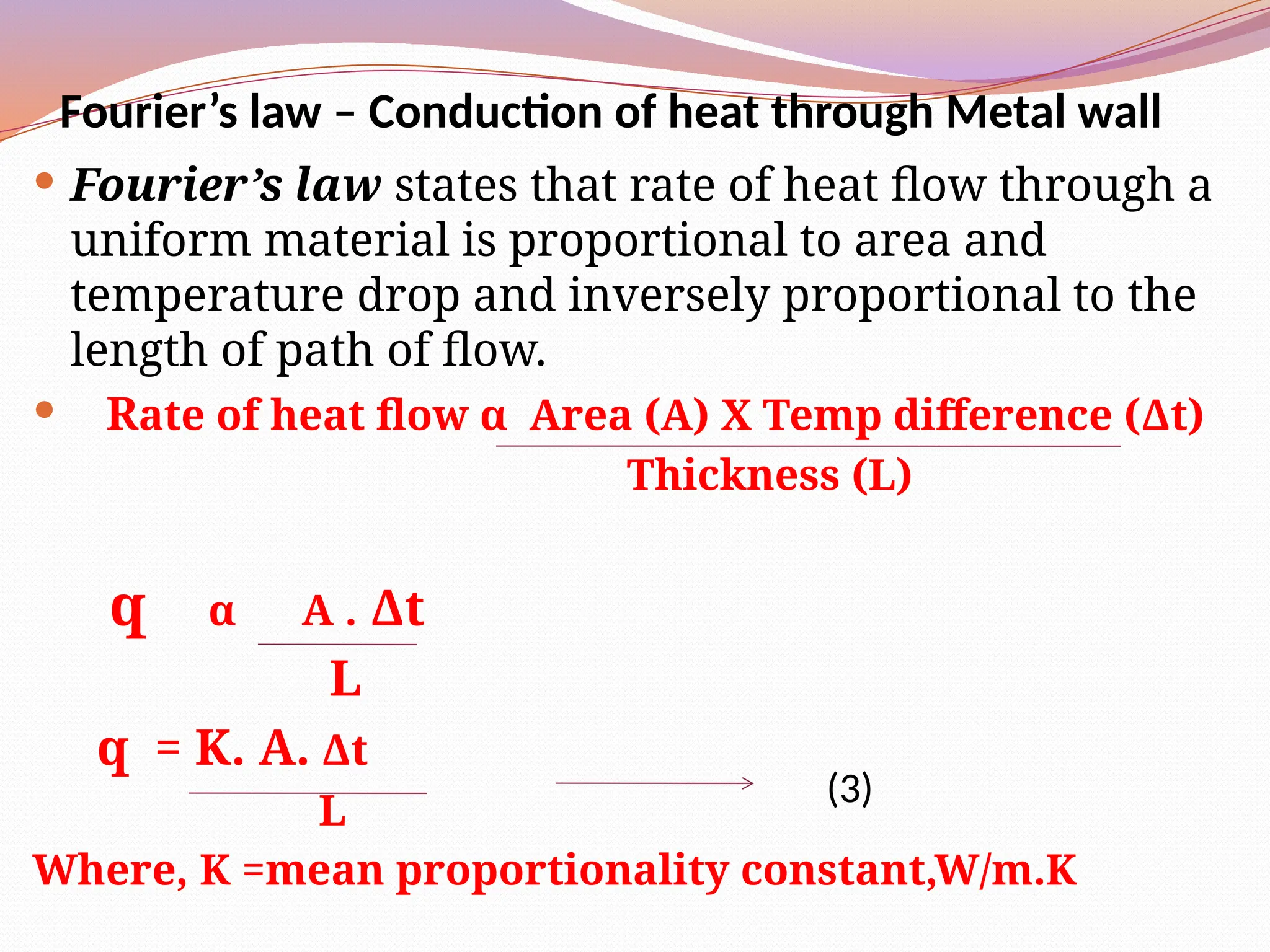
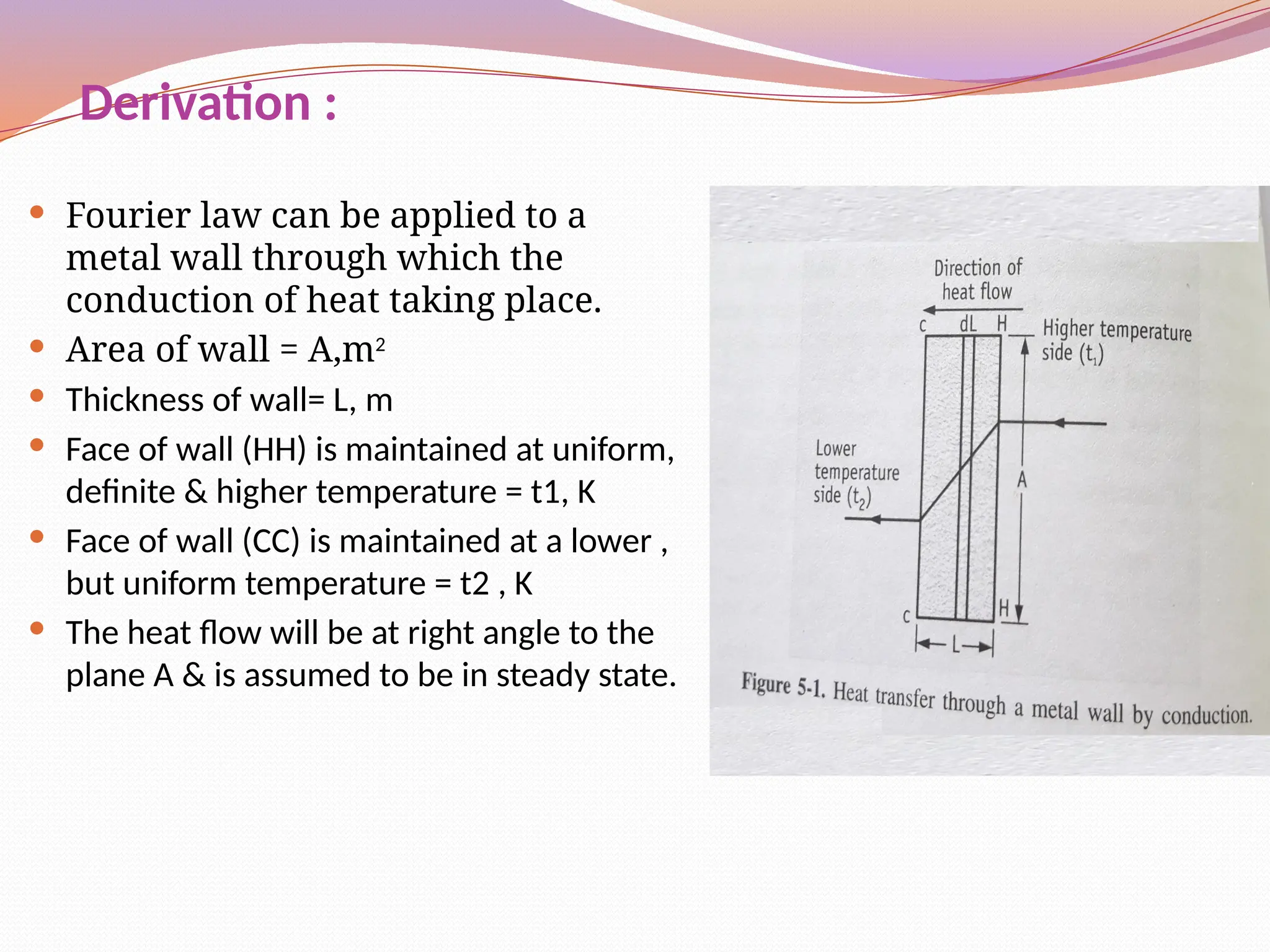
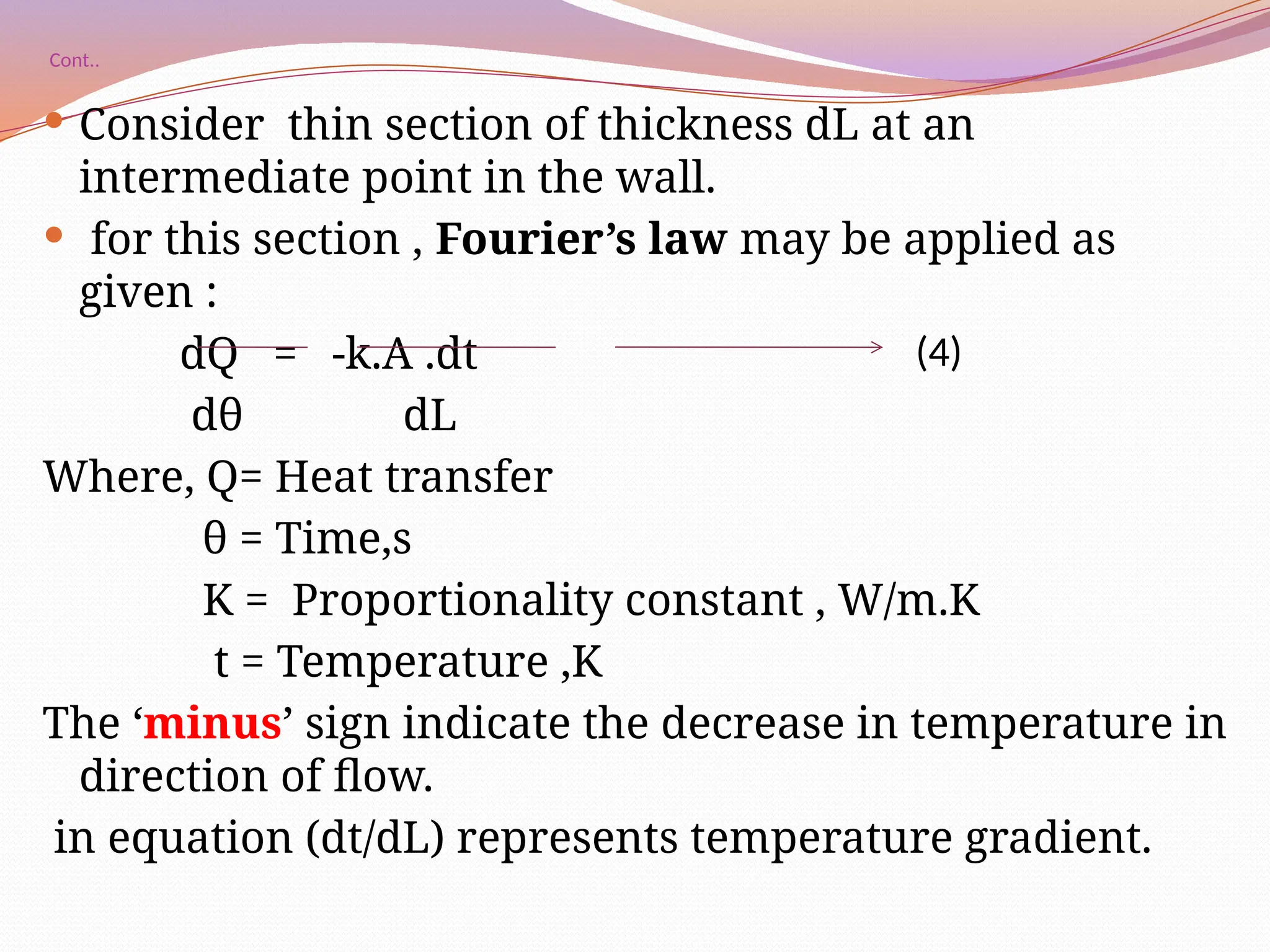
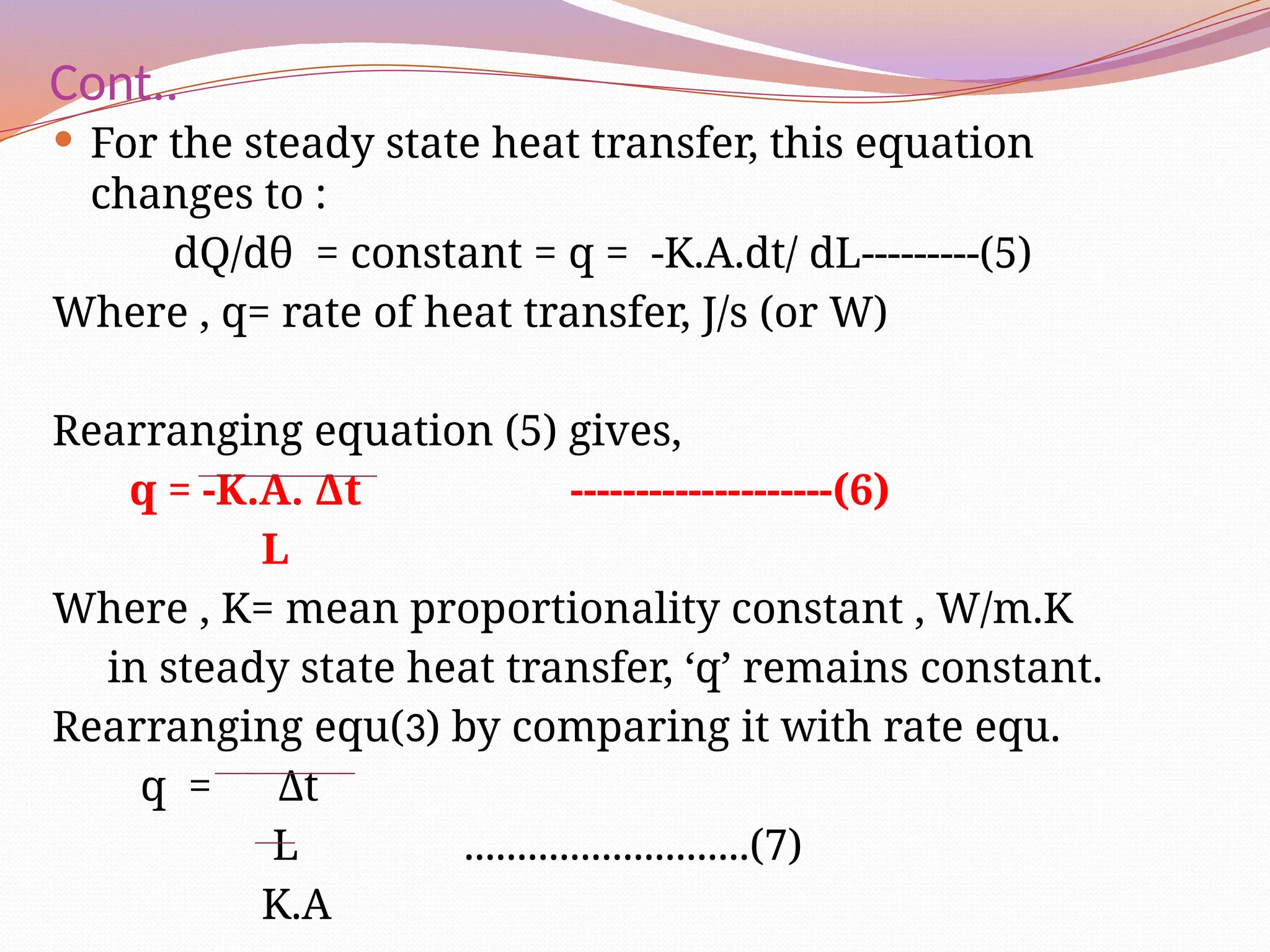
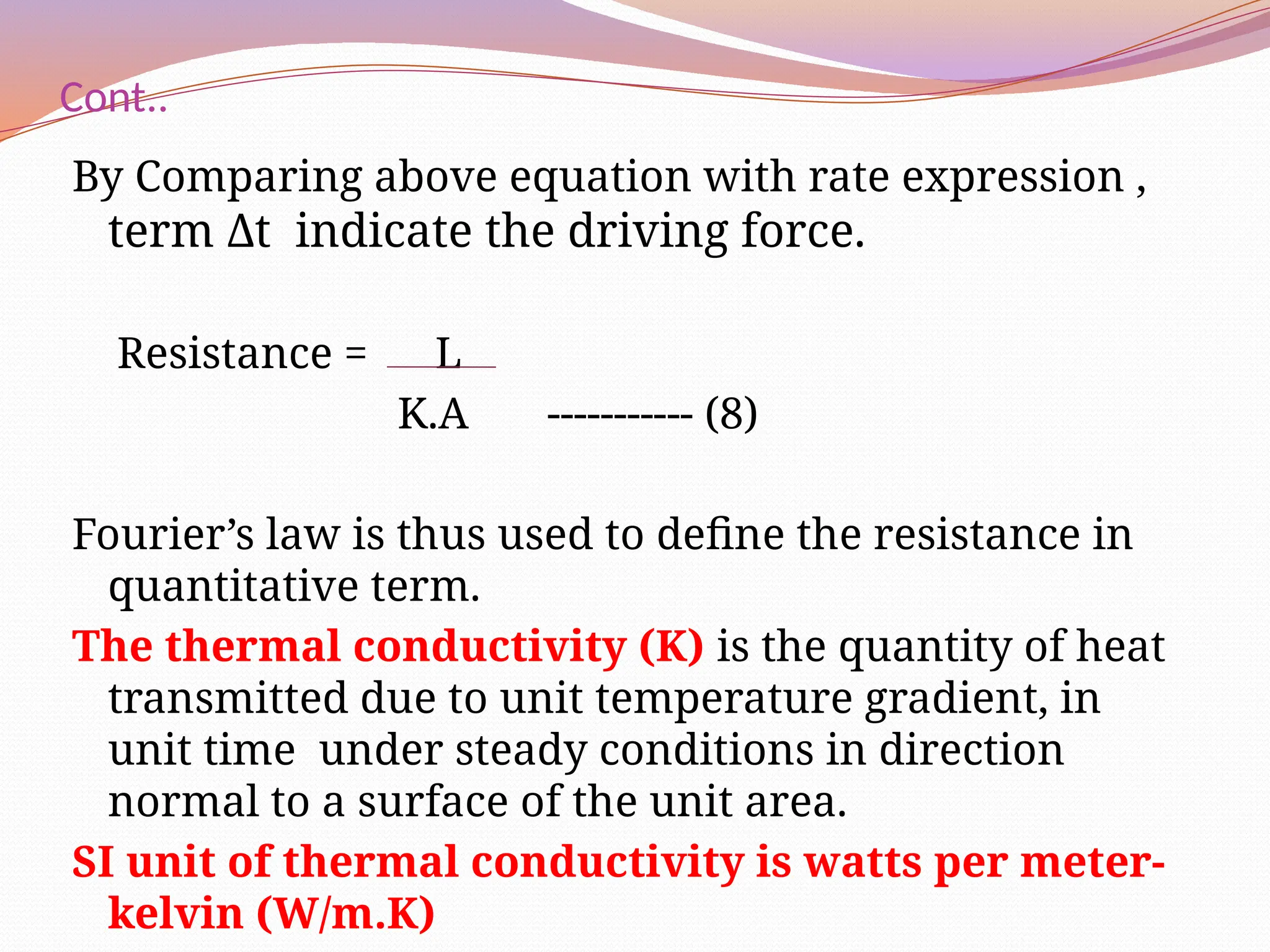
The document defines heat transfer as the movement of heat from high to low temperature systems, highlighting conduction, convection, and radiation as the three modes of heat transfer. It discusses various applications of heat transfer such as evaporation, distillation, and sterilization, as well as detailed explanations of conduction, convection, and radiation processes. Fourier's law is introduced to explain the quantitative aspects of heat conduction, demonstrating how the rate of heat flow is influenced by temperature differences and material properties.