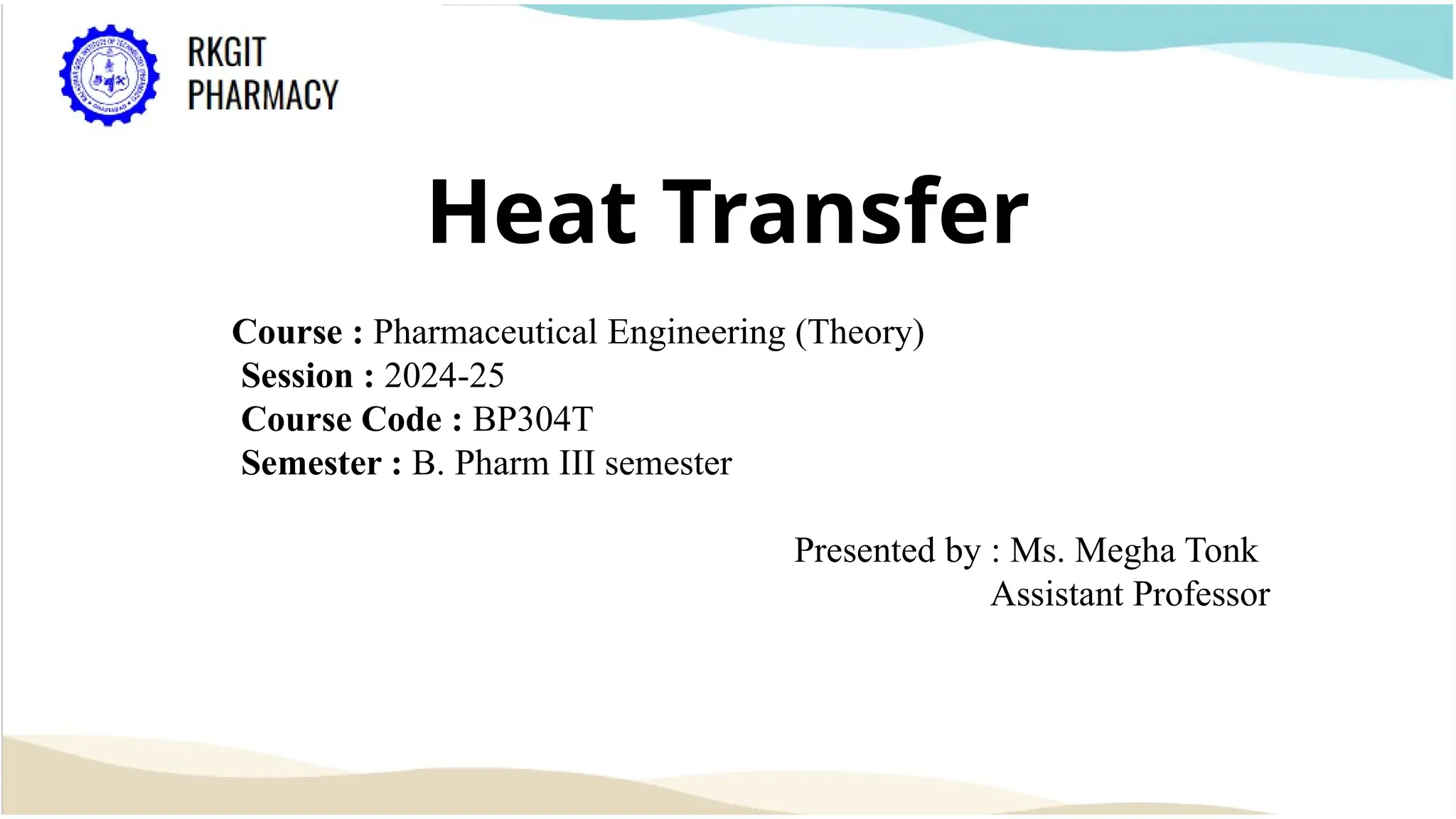
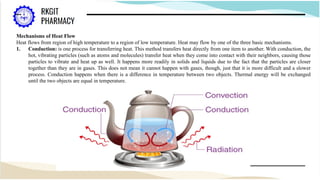
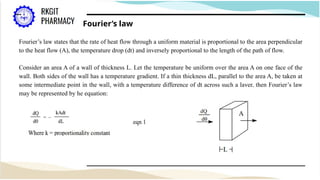
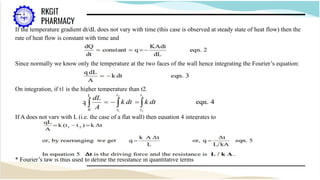
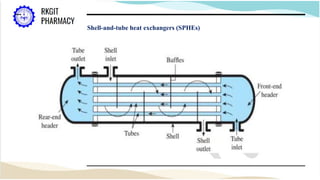
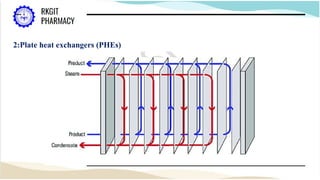
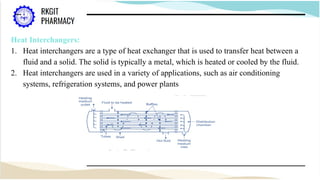
The document outlines a heat transfer course designed for pharmaceutical engineering, detailing the mechanisms of heat transfer including conduction, convection, and radiation, as well as Fourier's law and the types of heat exchangers used in industry. It discusses objectives such as energy efficiency and applications in processes like crystallization, distillation, and sterilization. Additionally, it covers the classification of heat exchangers based on flow arrangements and construction materials.