Embed presentation
Downloaded 13 times

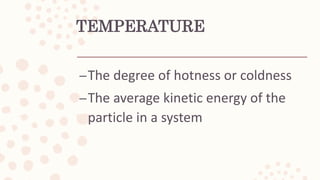


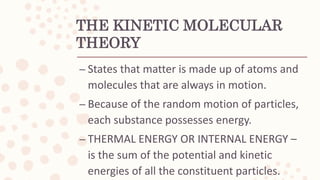


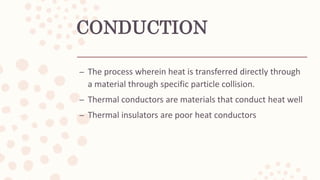



This document discusses heat, energy, temperature, and the three methods of heat transfer: conduction, convection, and radiation. It defines key terms like heat, thermal energy, temperature, and explains that heat transfer occurs due to temperature differences between objects. The kinetic molecular theory is also summarized, stating that matter is made up of particles in random motion, possessing kinetic energy.









