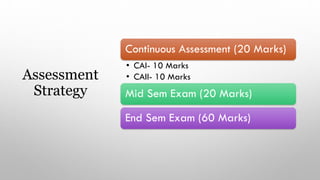
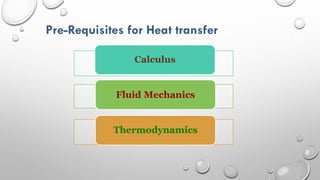
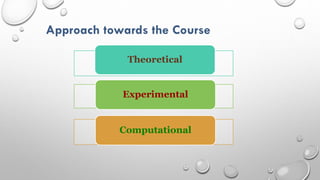
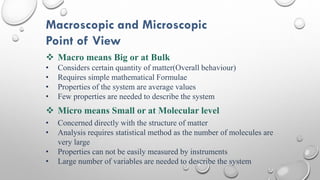
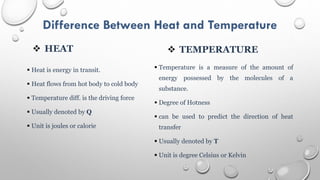
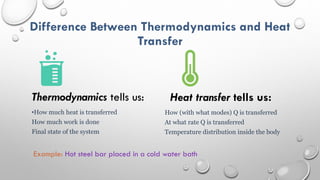
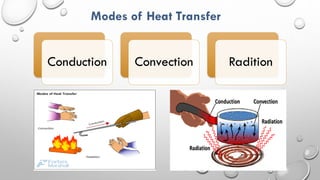
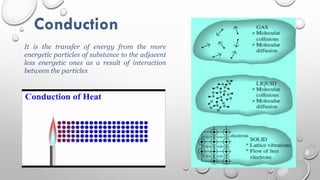
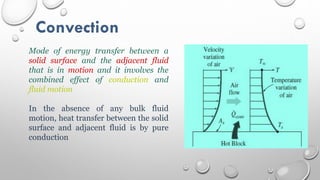
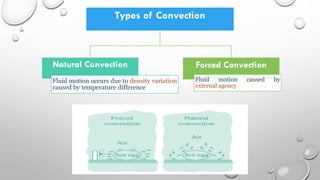
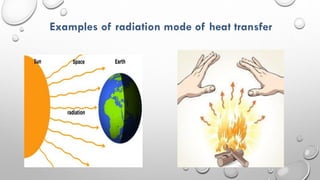
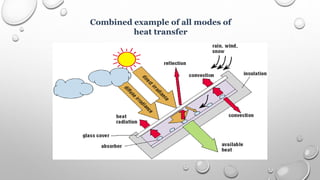
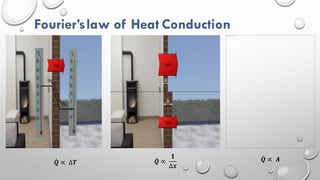
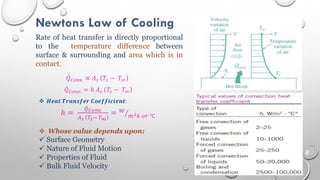
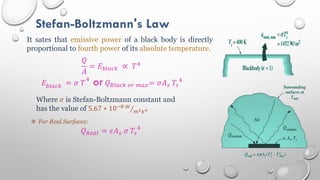
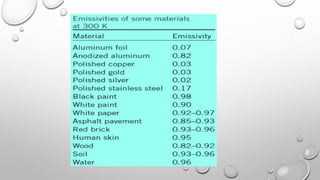
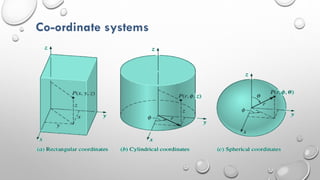
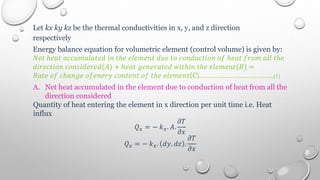
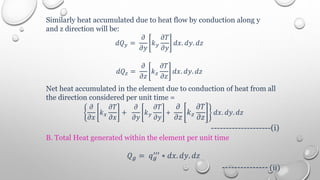
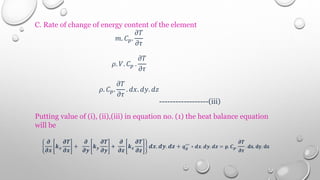
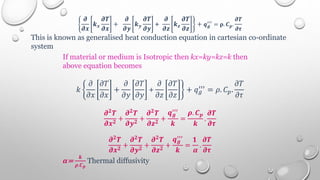
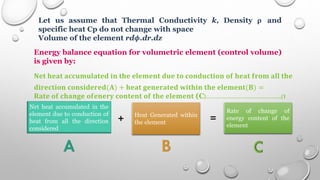
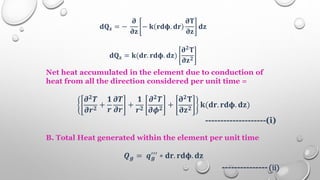
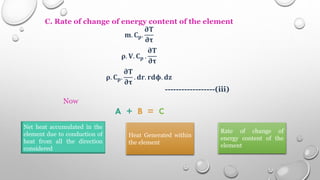
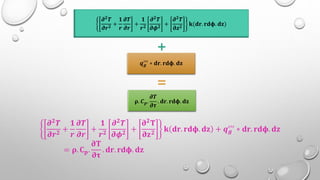
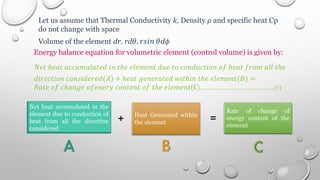
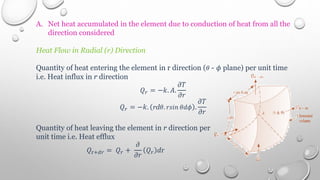
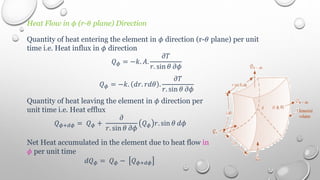
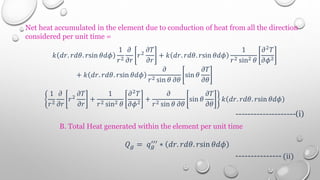
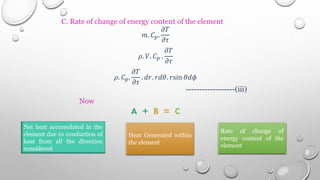
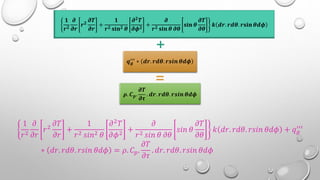
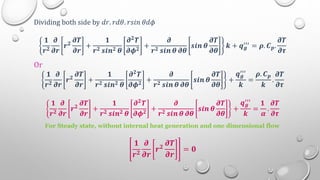
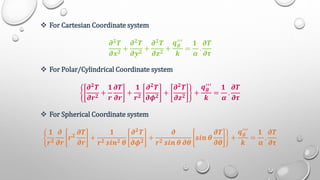
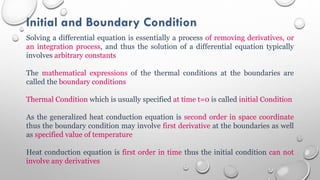
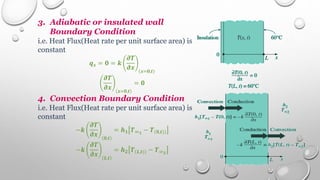
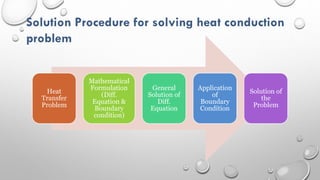
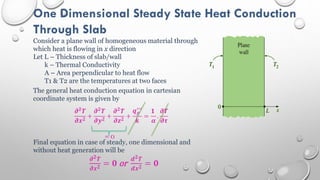
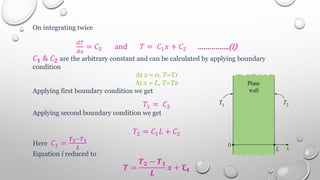
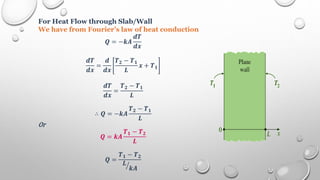
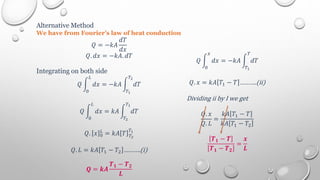
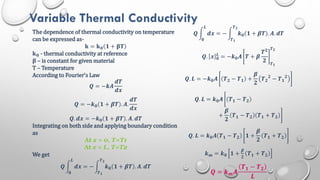
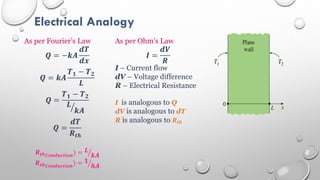
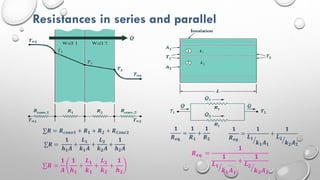
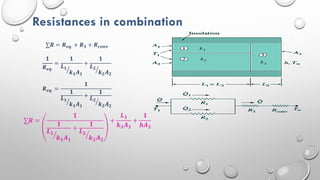
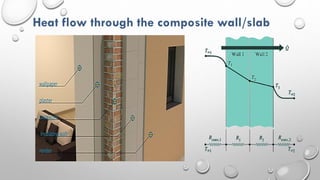
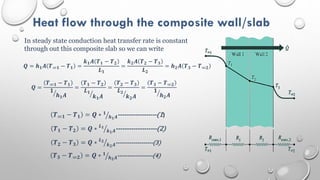
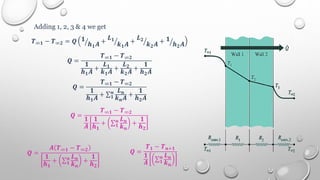
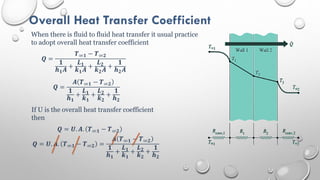
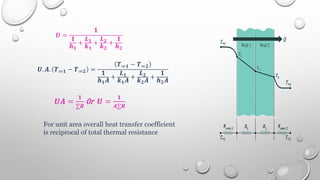
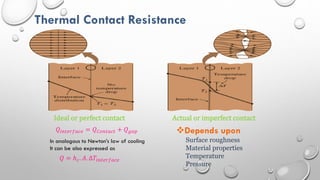
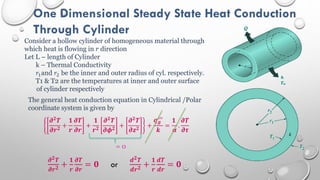
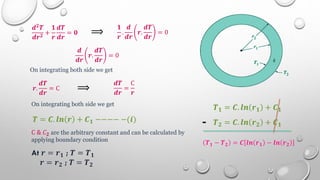
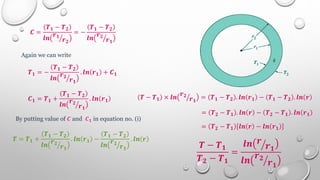
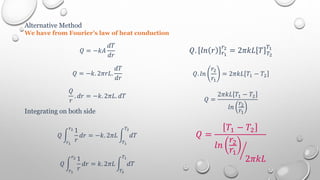
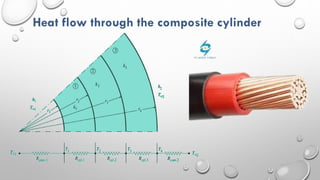
This document provides an overview of a course on heat transfer. The course is divided into 5 units that cover topics such as heat conduction, convection, radiation, and heat exchangers. Assessment includes continuous assessments, midterm and final exams. The course aims to explain heat transfer laws and analyze heat transfer problems involving various geometries and conditions. Key modes of heat transfer covered are conduction, convection, and radiation.