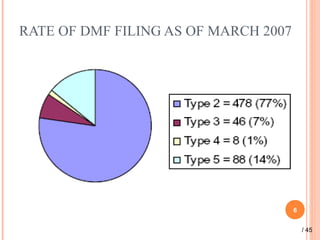
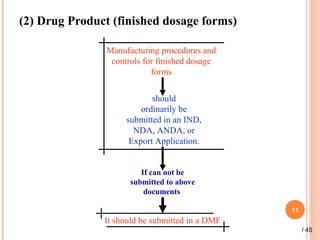
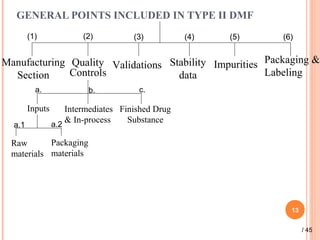
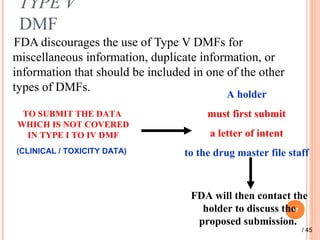
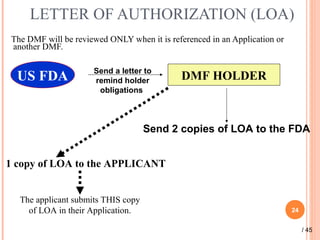
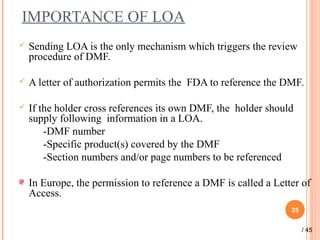
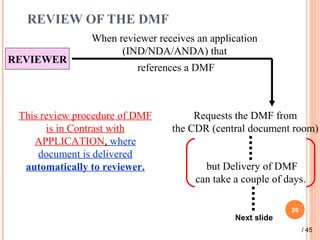
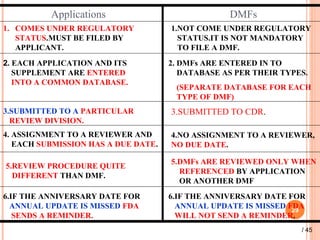
The document provides a comprehensive overview of Drug Master Files (DMFs), detailing their types, submission processes, and regulatory obligations. It highlights the specifics of each DMF type, outlines the responsibilities of DMF holders, and discusses the review procedures conducted by the FDA. Additionally, it covers the importance of annual updates and the potential retirement of inactive DMFs.