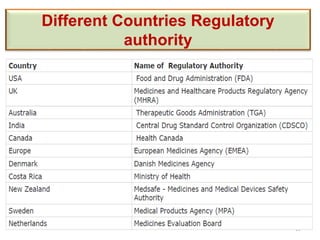
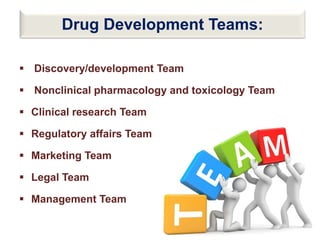
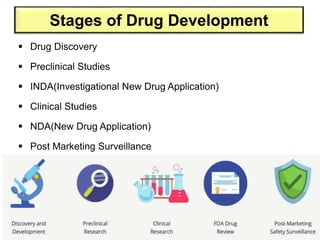
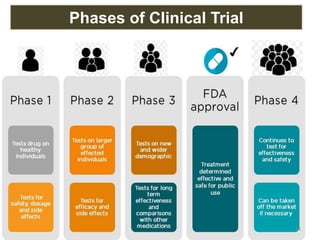
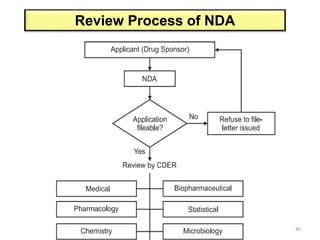
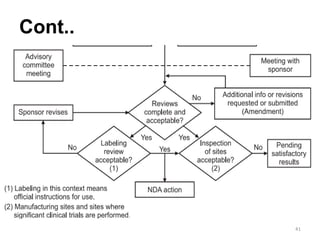
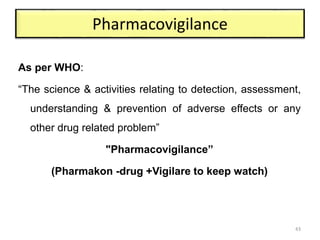
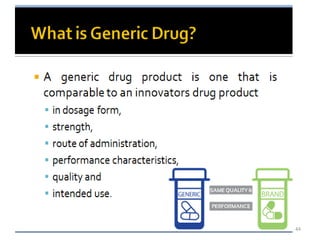
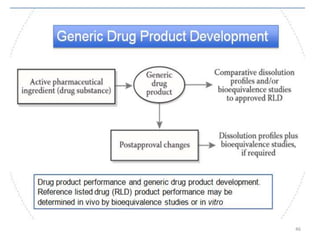
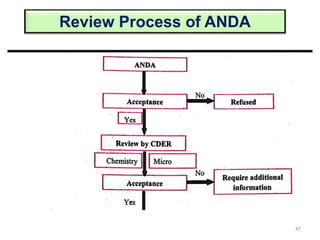
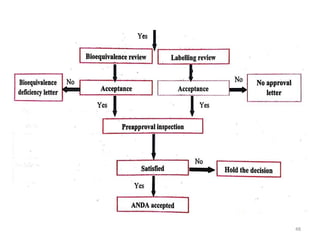
The document discusses the field of regulatory affairs, which is essential for ensuring public health by overseeing the safety and efficacy of pharmaceuticals and various health products. It provides an overview of historical incidents that necessitated strict drug regulations and outlines the roles and responsibilities of regulatory affairs professionals and departments. Additionally, it details the stages of drug development from preclinical testing to marketing authorization and emphasizes the importance of compliance with regulatory standards.