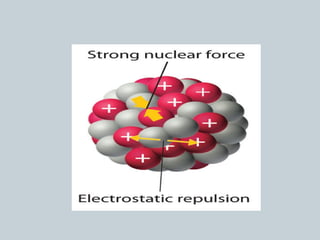
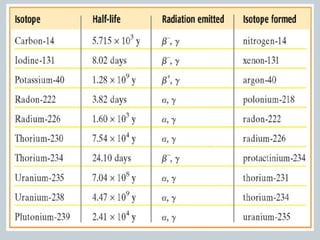
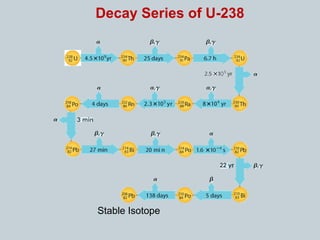
The document discusses nuclear stability and decay, highlighting that out of over 1500 known isotopes, only 264 are stable and the stability is influenced by the neutron-to-proton ratio. It explains the band of stability and the importance of neutrons in countering electrostatic repulsion among protons, with no stable isotopes existing beyond atomic number 83. Additionally, it covers half-life as the time required for half of a radioactive sample to decay, using examples like uranium-238 and phosphorus-32 to illustrate decay rates and calculations.