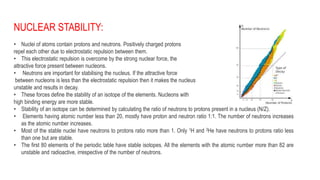
The document discusses several concepts related to nuclear stability and radioactive decay:
1) Nuclei are stabilized by the strong nuclear force between nucleons that overcomes electrostatic repulsion between protons. Stability depends on the ratio of neutrons to protons and binding energy of nucleons.
2) The law of radioactive decay states that the number of nuclei decaying per unit time is proportional to the total number of nuclei and follows an exponential decay function.
3) Half-life is the time for half the nuclei in a sample to decay and can be calculated from the decay constant. Mean life is the average lifetime of all nuclei in a sample. It is related to half-life and decay constant.