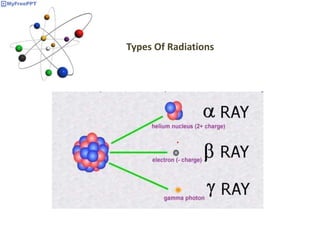
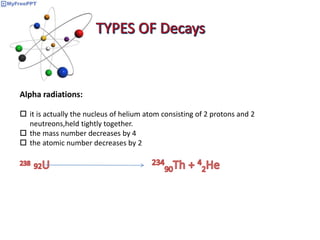
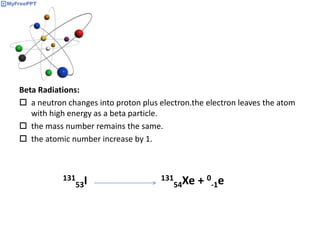
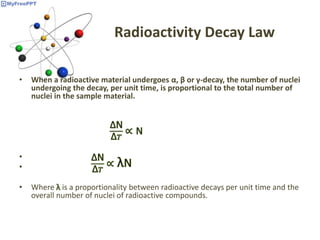
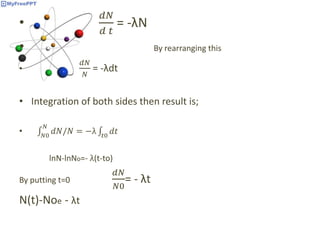
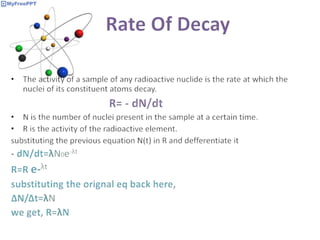
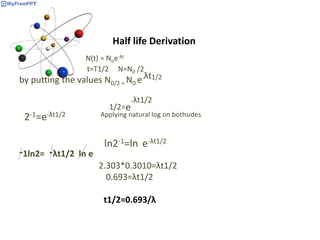
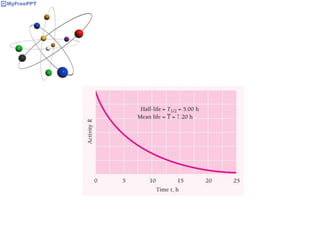
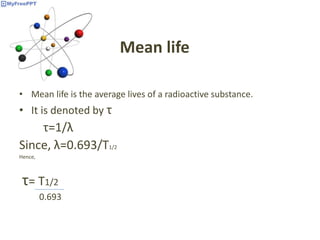
Radioactive decay occurs when the nucleus of an atom spontaneously disintegrates. Heavy atoms like uranium, thorium, radium, and polonium undergo this process, emitting alpha, beta, or gamma radiation. The rate of radioactive decay follows first-order kinetics, meaning the rate of decay is proportional to the amount of radioactive material. This allows scientists to define quantities like half-life, the time it takes for half of a radioactive sample to decay, and mean life, the average lifetime of radioactive atoms, which is equal to the half-life divided by the natural logarithm of two.