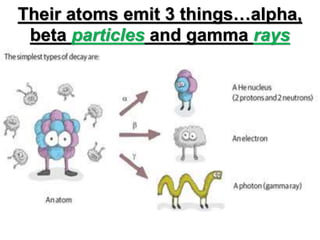
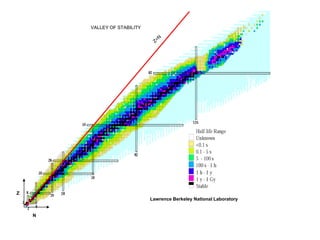
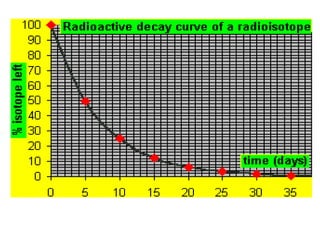
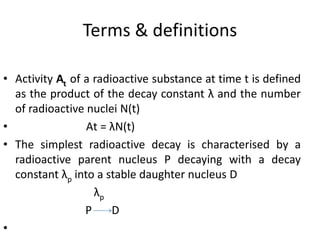
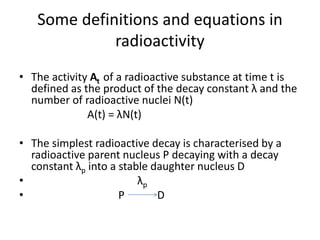
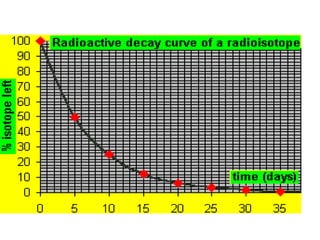
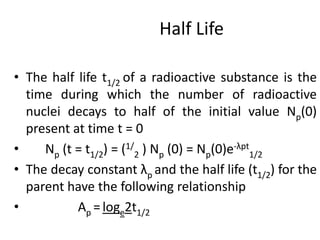
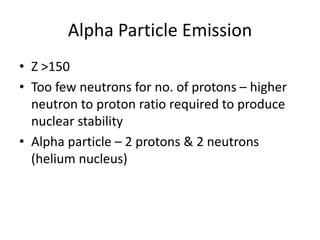
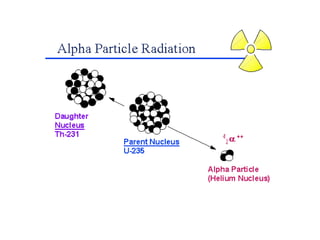
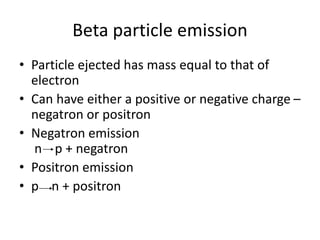
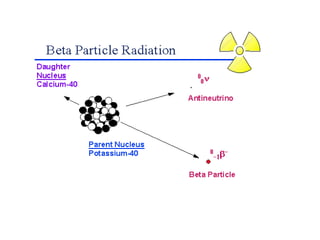
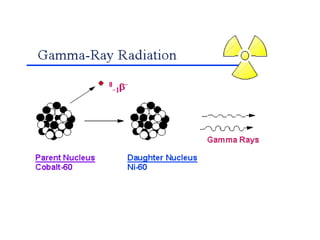
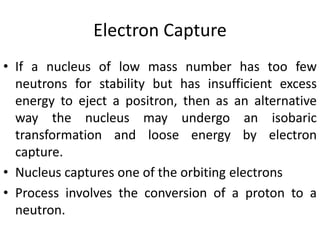
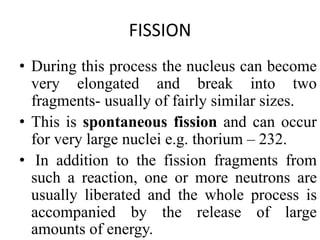
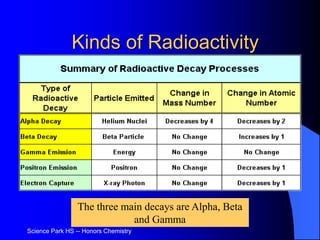
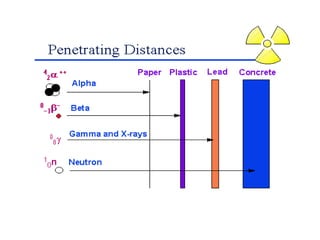
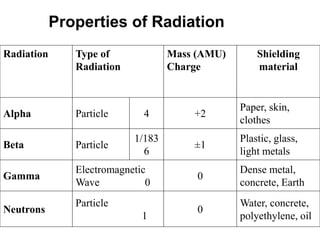
The document discusses the stability of atomic nuclei and different types of radioactive decay. It explains that nuclei are stable when the nuclear binding forces outweigh the electrostatic repulsion between protons. Unstable nuclei undergo radioactive decay through processes like alpha, beta, or gamma emission to become more stable. The activity of a radioactive sample decreases exponentially over time according to the half-life. Different modes of decay include alpha emission by nuclei with too few neutrons, beta emission changing the number of protons and neutrons, and gamma emission releasing excess energy.