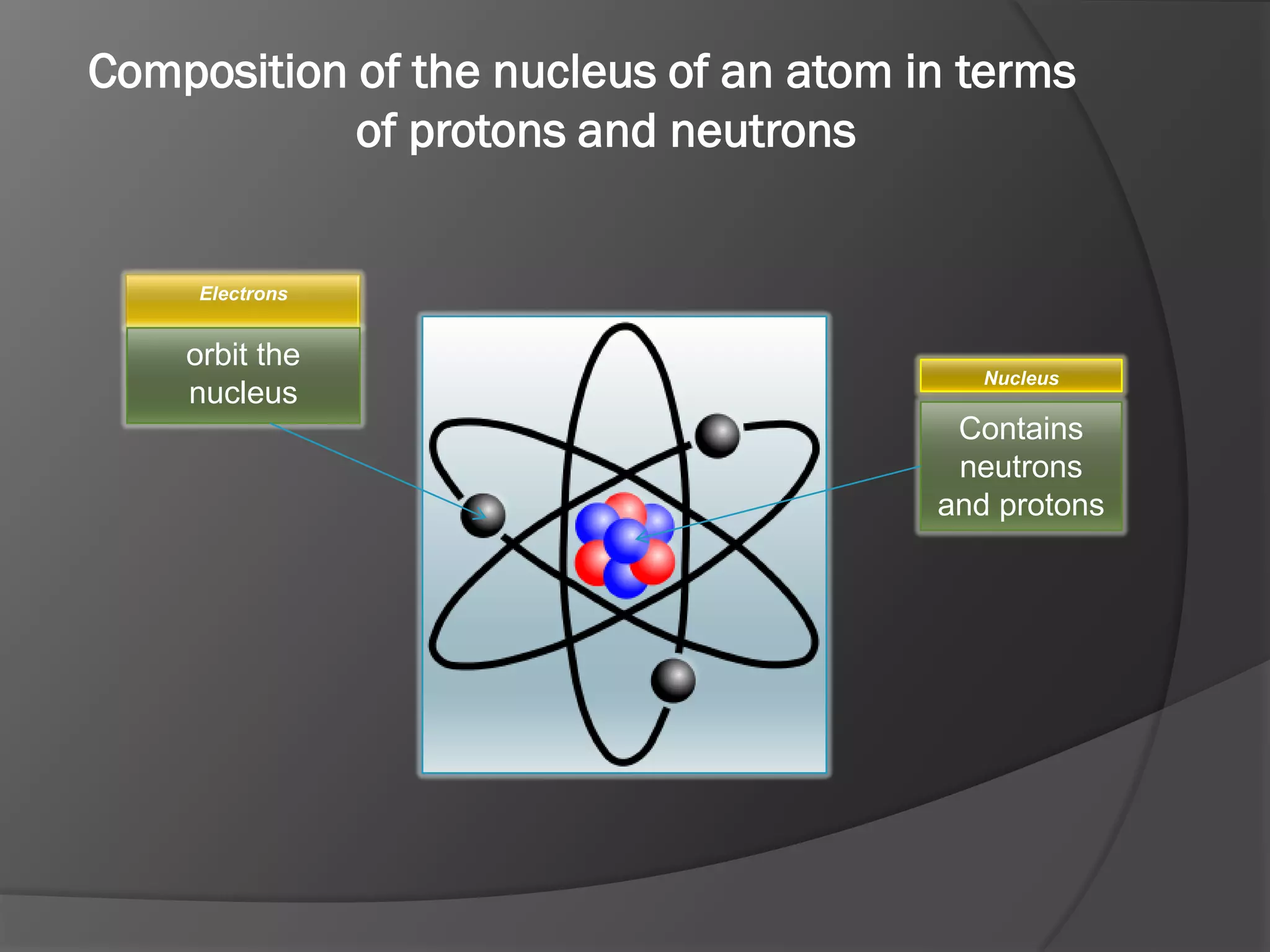
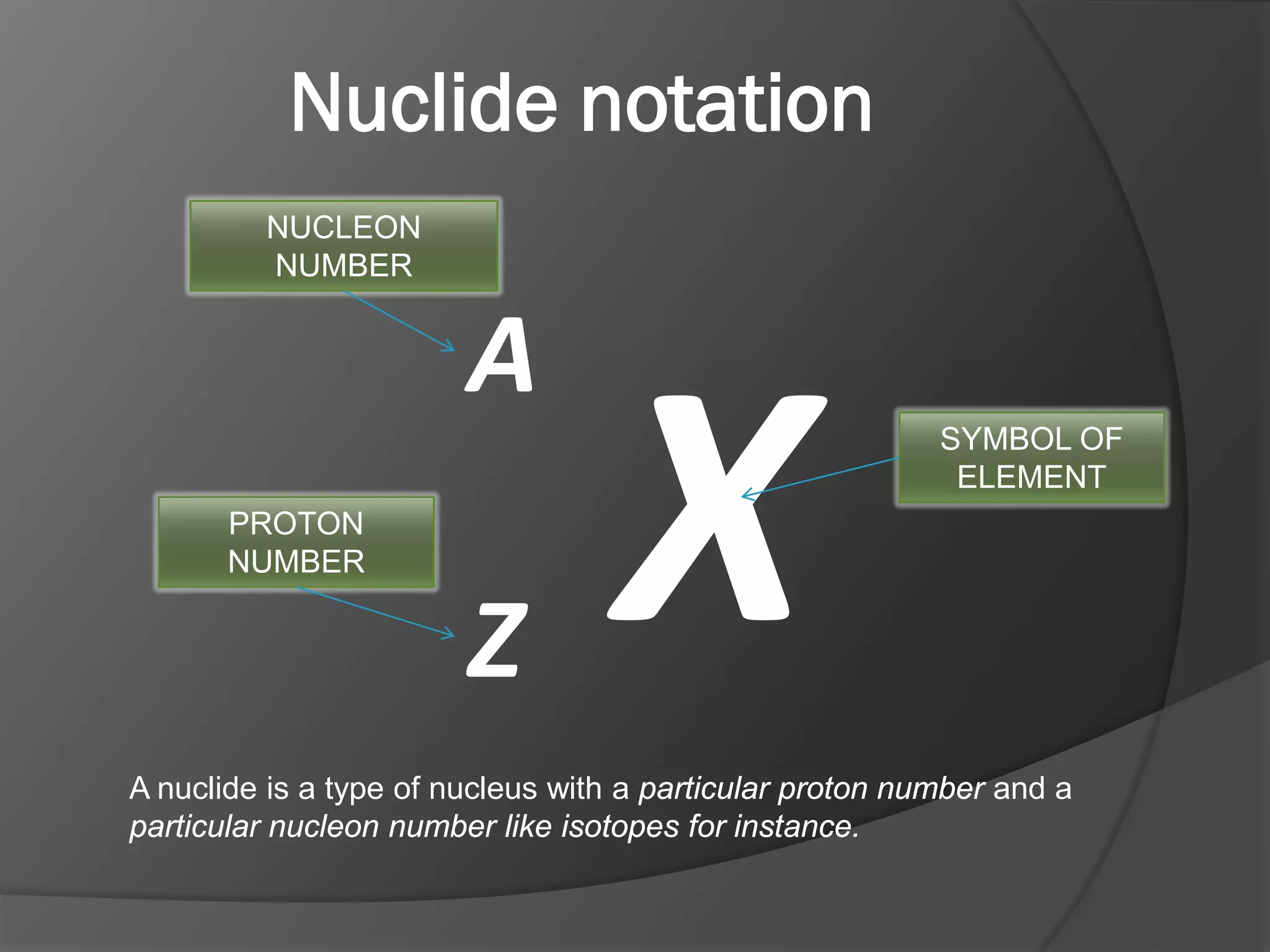
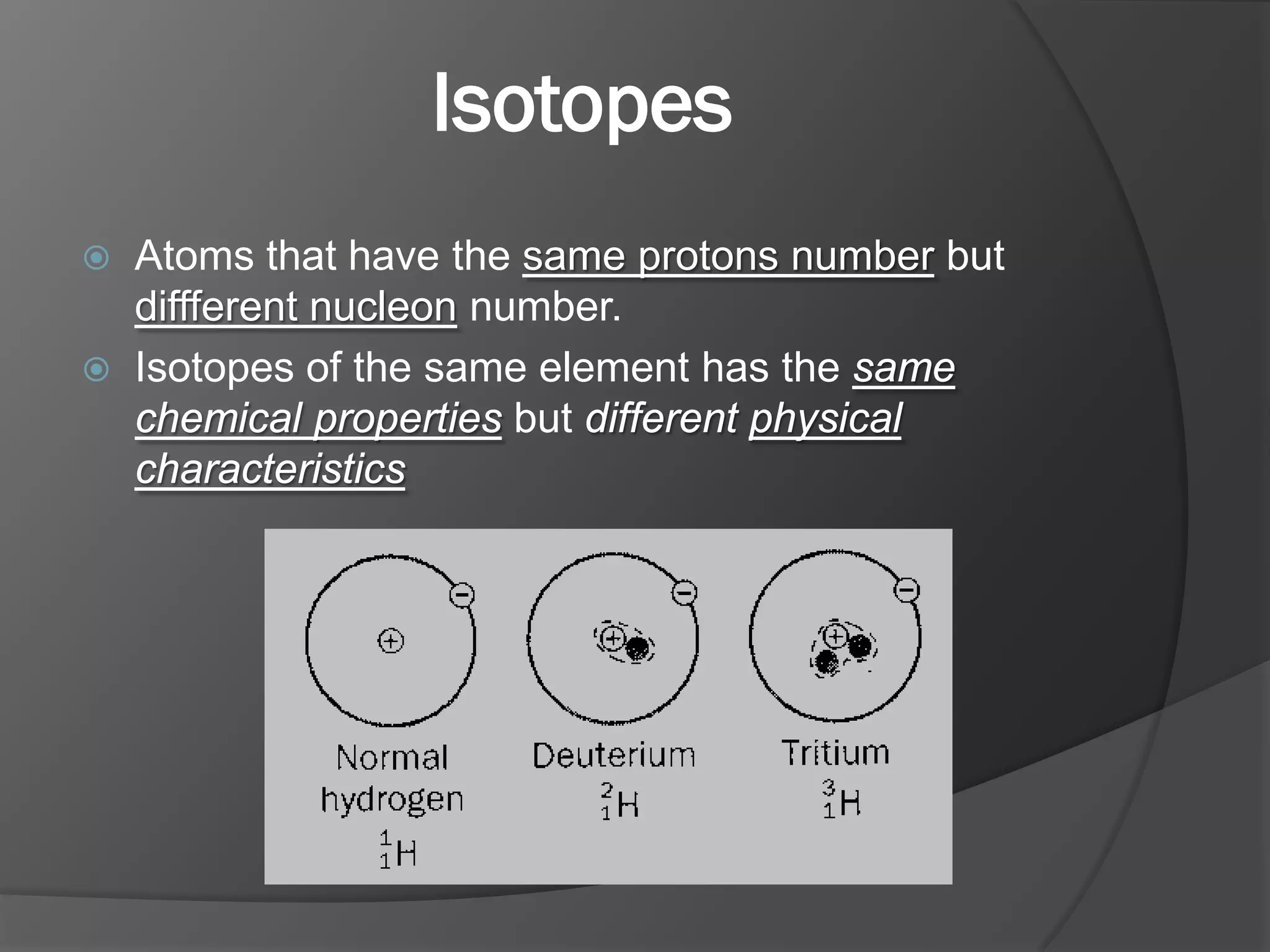
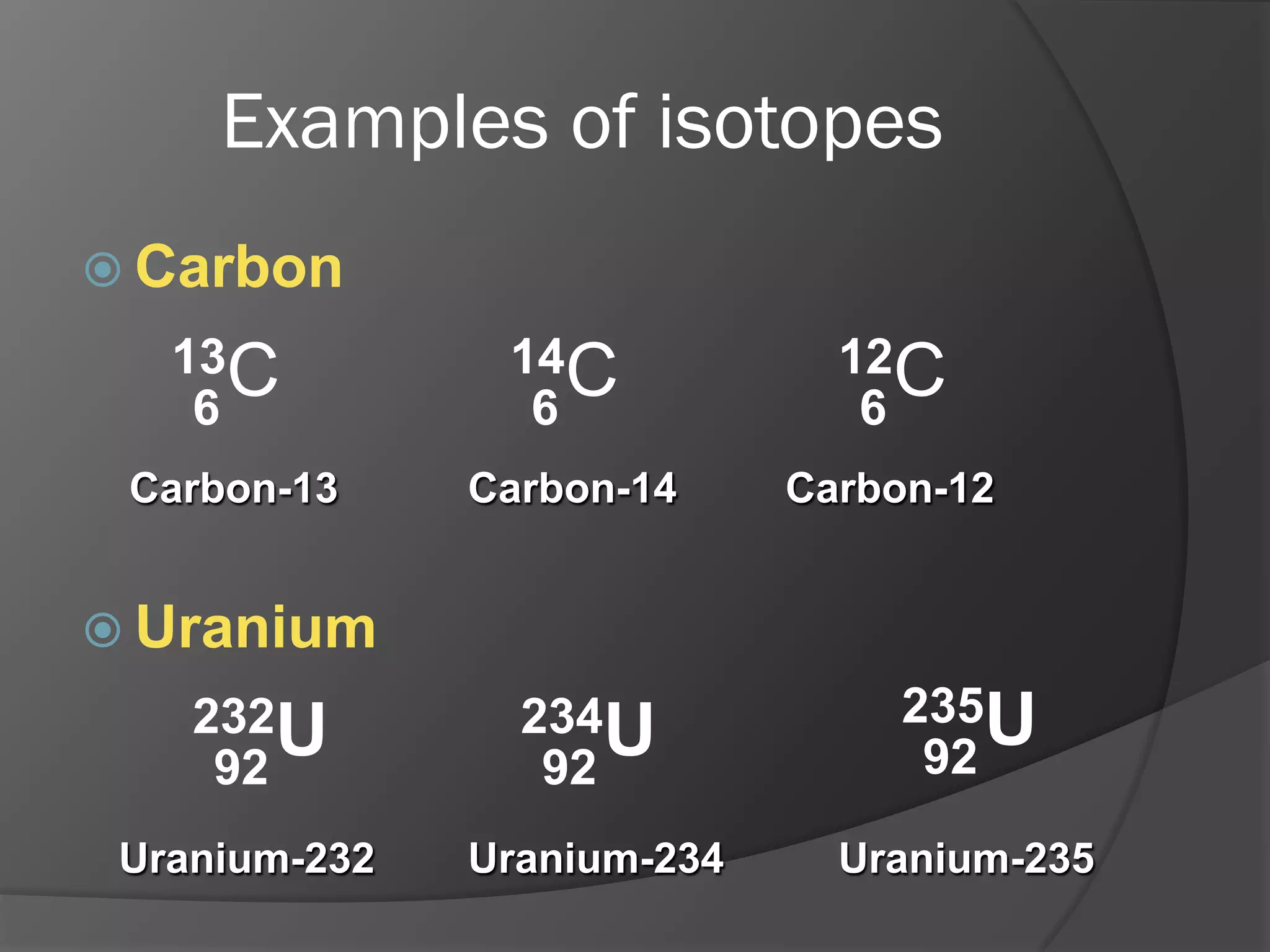
The document discusses key concepts about the nucleus of an atom including its composition of protons and neutrons. It defines important terms like proton number (Z), nucleon number (A), and nuclide notation. Isotopes are introduced as atoms of the same element that have the same number of protons but different numbers of neutrons. Examples are provided to demonstrate calculating nucleon numbers and identifying isotopes.