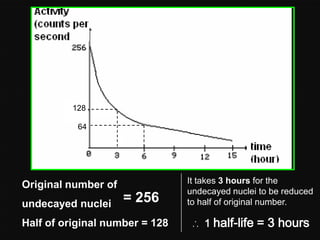
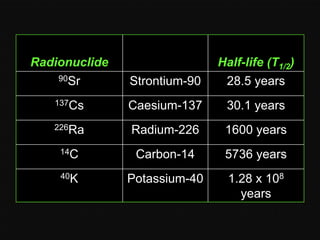
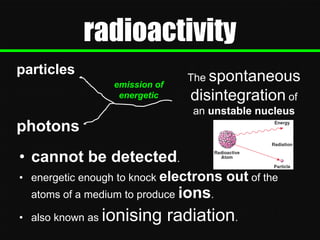
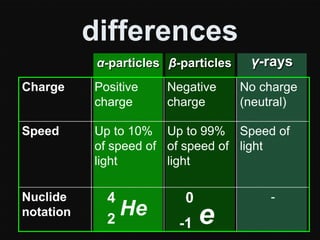
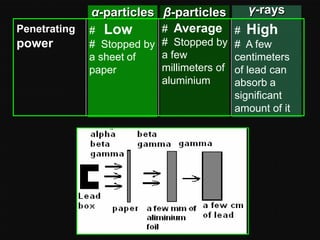
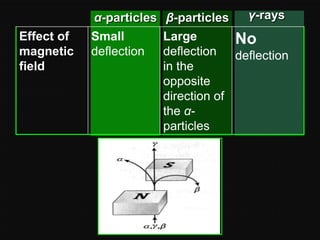
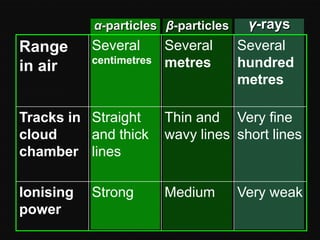
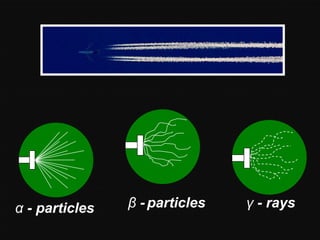
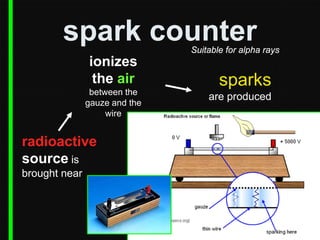
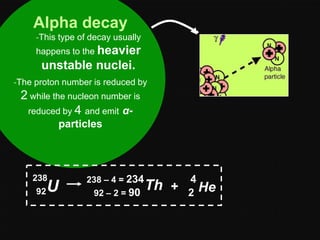
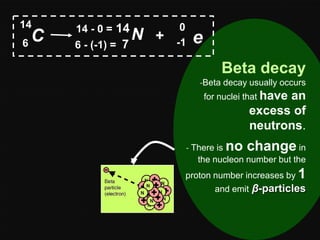
Radioactive decay occurs when an unstable nucleus spontaneously disintegrates by emitting particles or electromagnetic waves. There are three main types of radioactive emissions: alpha particles, beta particles, and gamma rays. Each type has distinct properties in terms of charge, speed, penetrating power, and interaction with electric and magnetic fields. Radioactive decay continues in a nucleus until it reaches a stable configuration. The rate of decay is random but consistent, allowing the calculation of half-life, the time for half the radioactive nuclei to decay. Common radioactive isotopes and their half-lives are listed.






![Gamma decay
-occurs when an unstable nucleus
releases its excess energy [ high
frequency electromagnetic
waves ] called γ-rays.
-no change in proton number and
nucleon number but emit γ-rays
-A nucleus that undergoes alpha or beta
decay may
60
Co
27
also emit γ-rays.
60
27
Co +
γ](https://image.slidesharecdn.com/azra-radioactivedecay-140226091658-phpapp02/85/Azra-radioactive-decay-20-320.jpg)