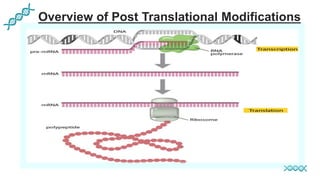
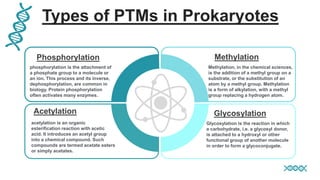
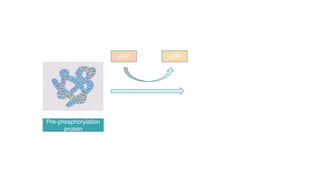
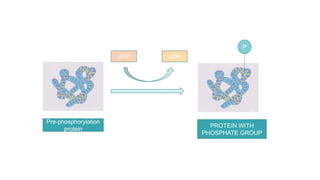
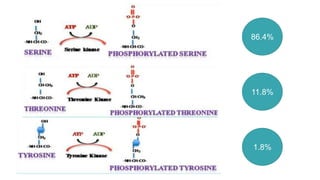
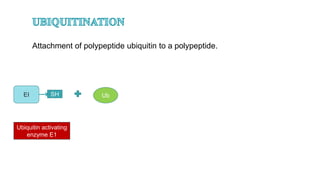
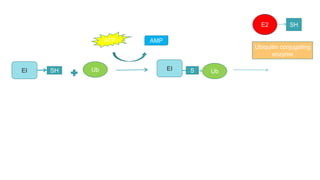
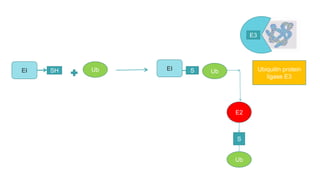
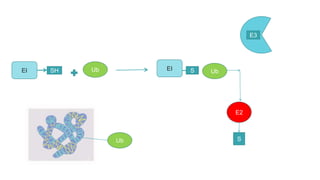
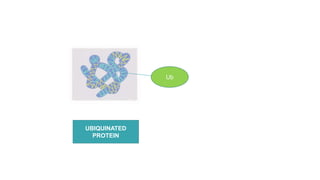
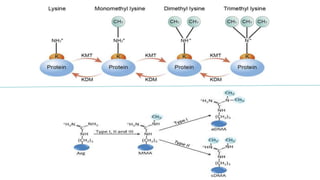
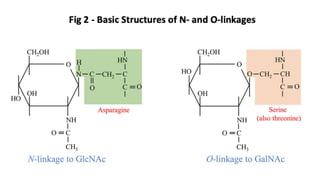
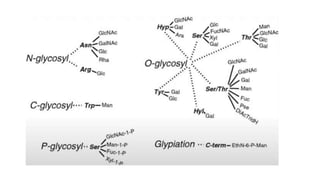
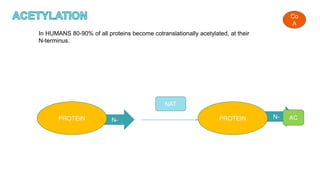
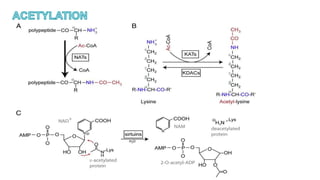
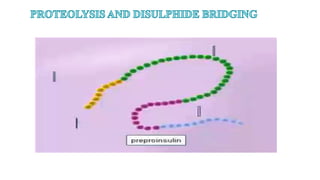
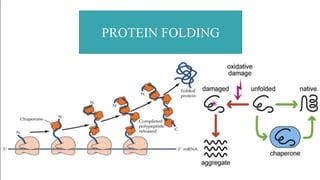
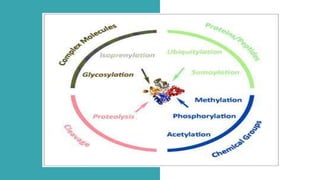
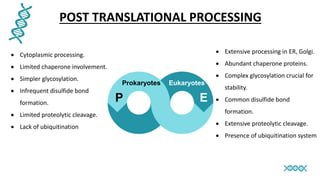
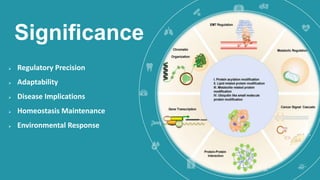
This document discusses post-translational modifications (PTMs) in prokaryotes and eukaryotes. It begins with an introduction to PTMs, noting that they are enzymatic modifications of proteins that can change properties like activity and stability. Sections then discuss specific PTMs and their roles in prokaryotes and eukaryotes, as well as techniques to study them. Comparisons are made between prokaryotic and eukaryotic PTMs and their significance. The conclusion states that PTMs shape protein functionality and influence cellular processes and disease pathways, playing a pivotal role in cellular responses.