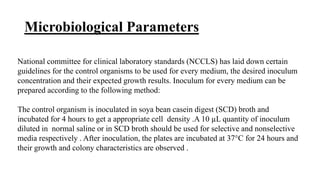
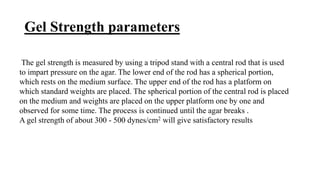
The document outlines essential practices and quality control measures in microbiology laboratories, emphasizing the importance of personnel training, laboratory environment, and media preparation. It details various parameters for quality assurance, including sterilization, physical characteristics, and microbiological standards. Finally, it highlights the significance of good laboratory practices (GLP) and the need for continuous monitoring and adherence to protocols to ensure reliable results.