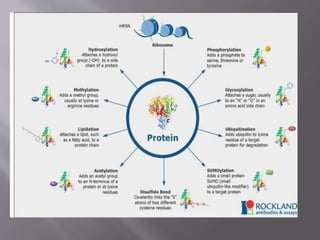
Post-translational modifications (PTMs) refer to any alterations made to proteins after their initial synthesis, such as modification of amino acid side chains. PTMs influence protein structure, stability, activity, and more. Common PTMs include phosphorylation, glycosylation, methylation, and hydroxylation. PTMs are important for proper protein folding, conferring stability, and regulating protein activity and function. They increase proteome diversity and complexity.