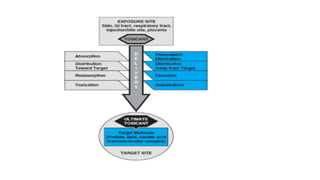
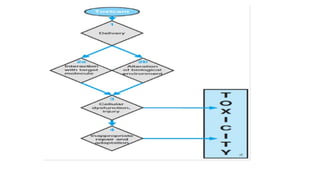
The document provides an overview of toxicology, highlighting the importance of toxicity testing in ensuring the safety of pharmaceuticals as per international regulations like OECD guidelines. It covers the history, principles, and applications of toxicology, detailing various types of toxicity assessments and the role of toxicologists in research and safety evaluations. Additionally, it discusses the risks associated with working in toxicology labs and emphasizes quality assurance in testing processes.