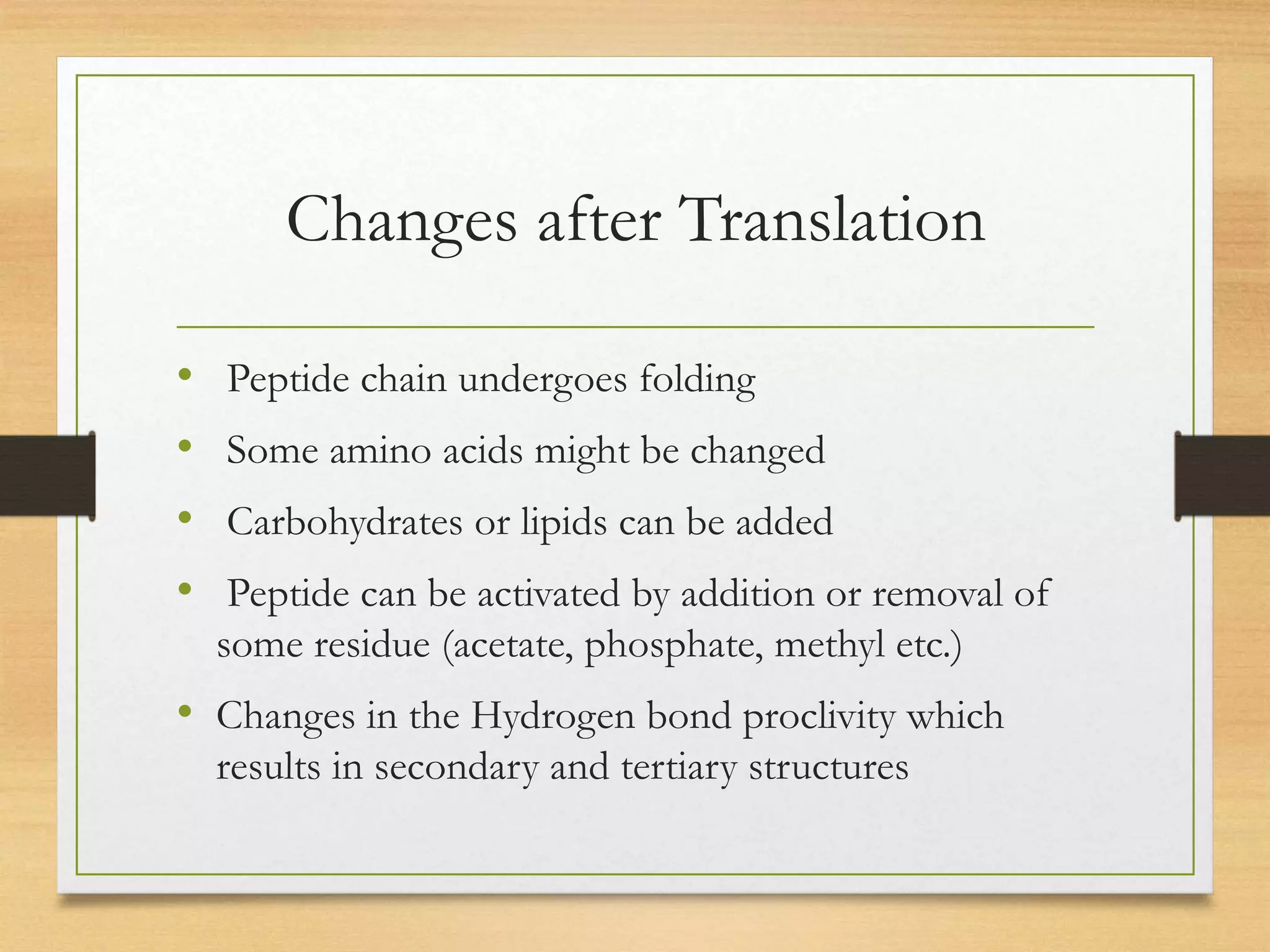
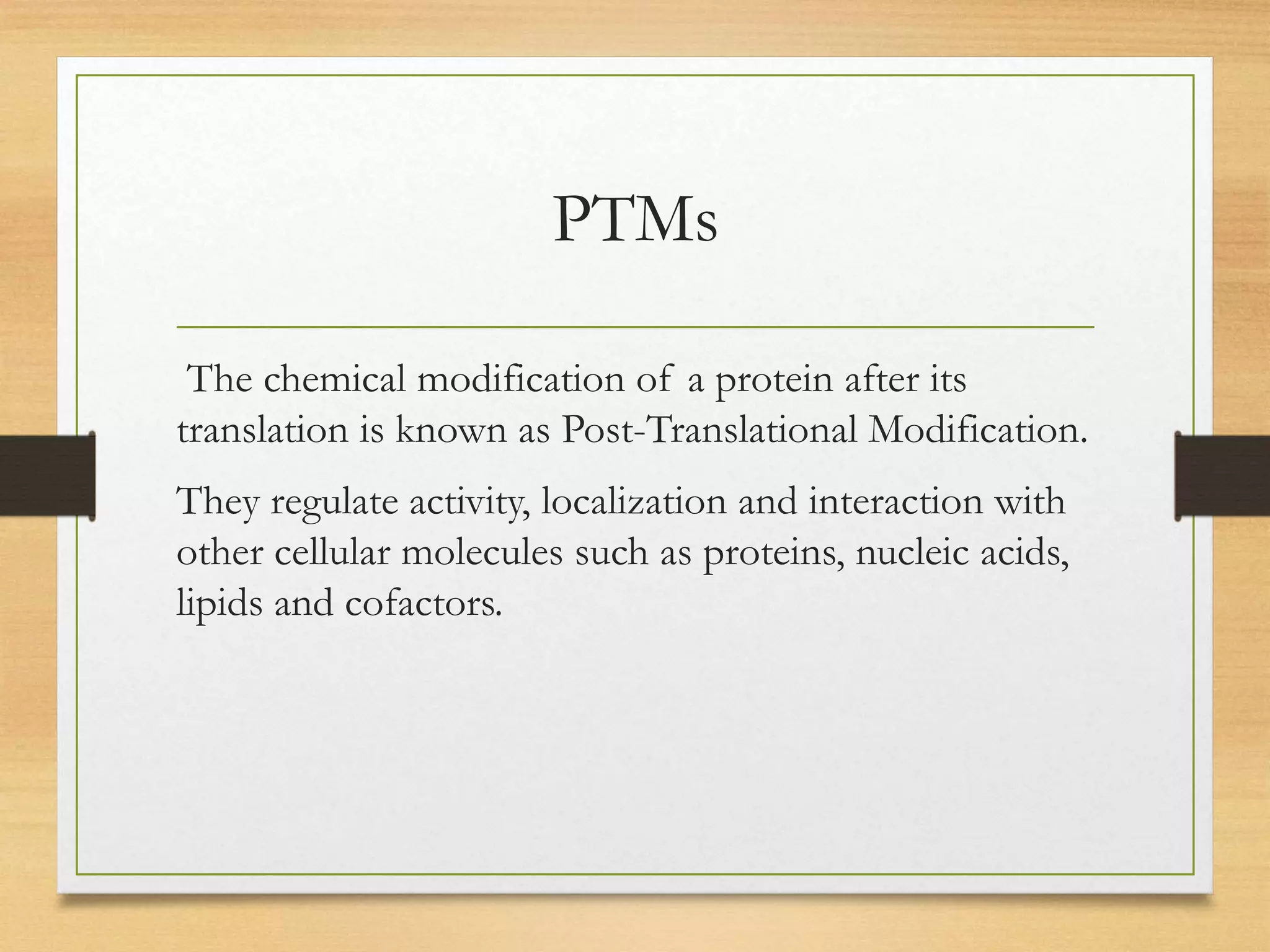
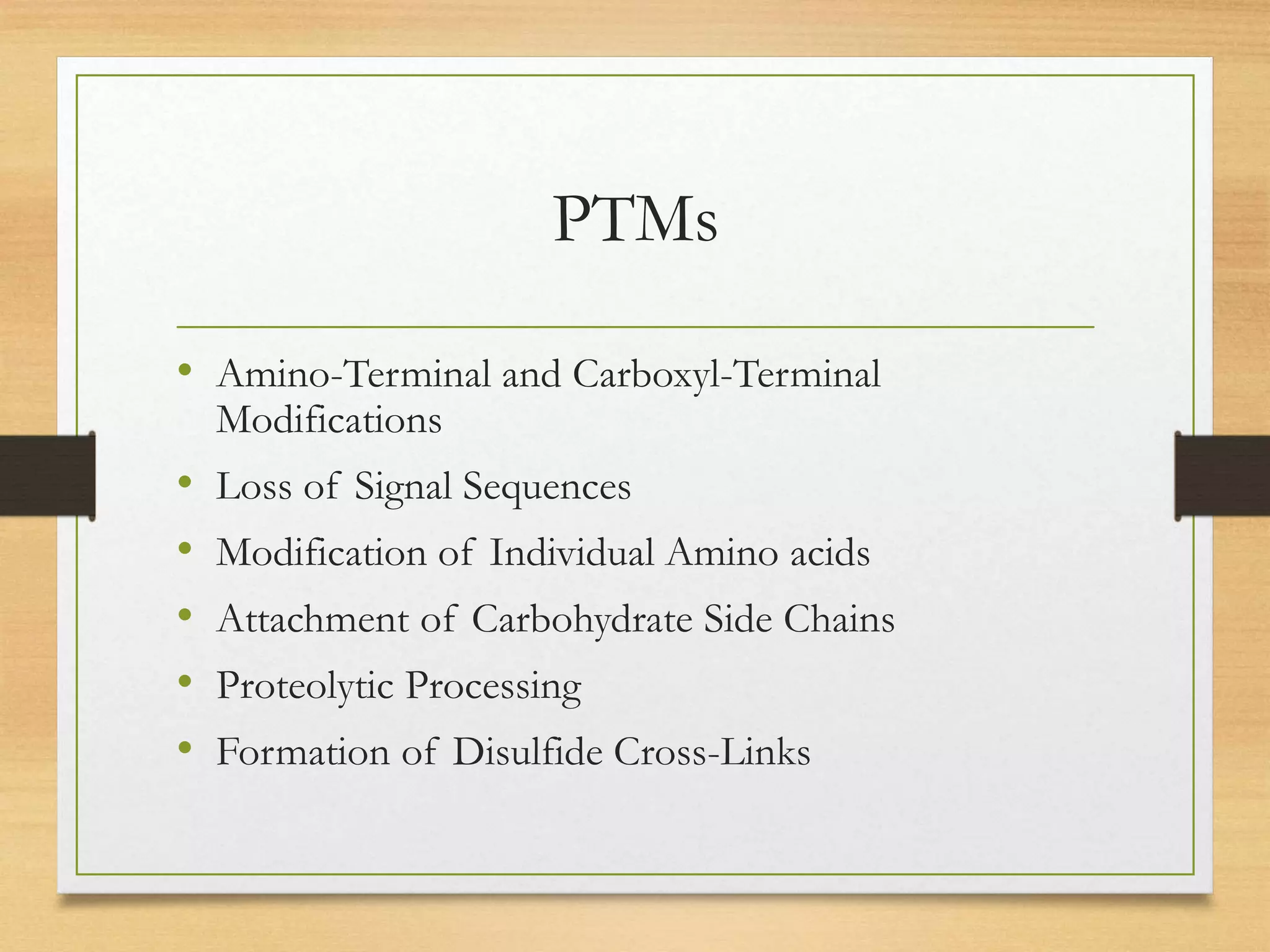
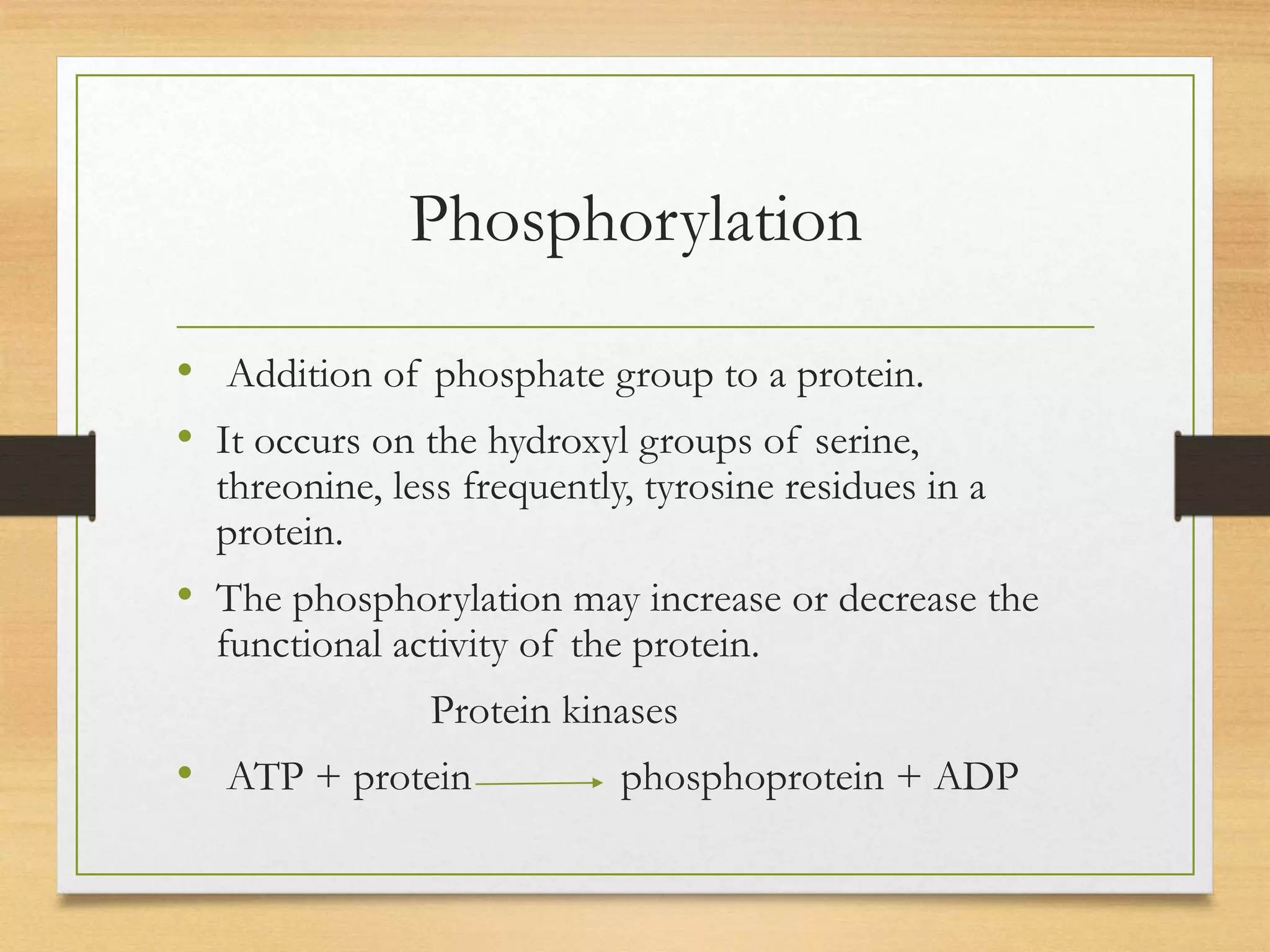
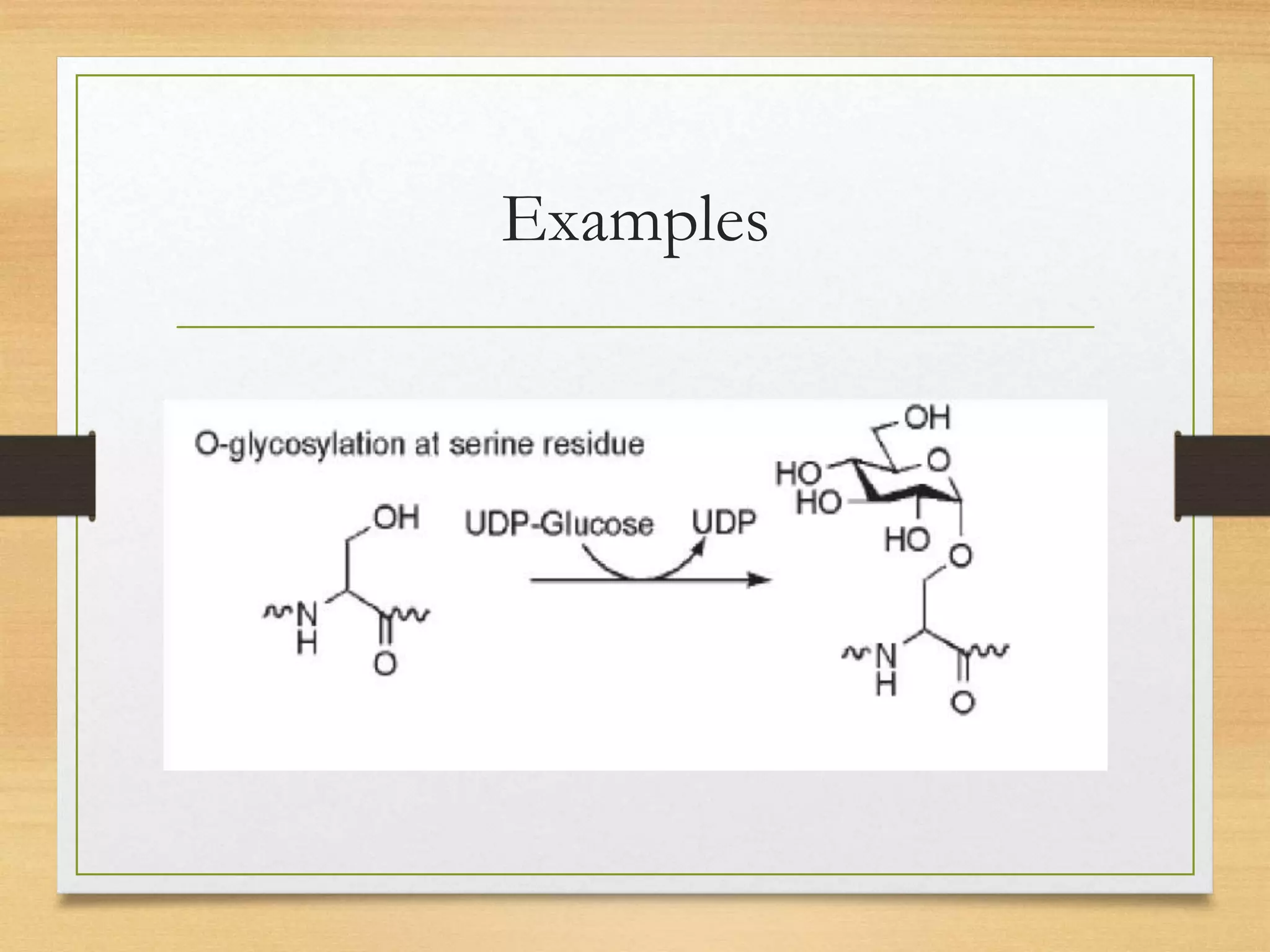
Post-translational modifications (PTMs) are chemical changes that occur to proteins after translation. PTMs regulate proteins' activity, localization, and interactions and allow identical proteins to have different functions in different cell types. Common PTMs include phosphorylation, glycosylation, ubiquitination, and proteolytic processing. PTMs are important for biological processes but can also contribute to disease when dysregulated.













![Progressive Systemic Sclerosis
(Scleroderma]
• A disease of the vascular and immune systems, and a
severe connective tissue disorder.](https://image.slidesharecdn.com/ptms-191127103518/75/post-translational-modification-20-2048.jpg)