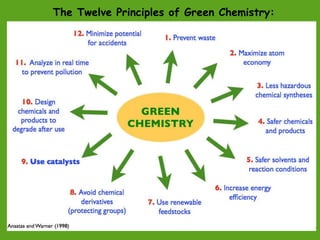
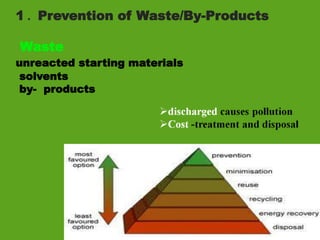
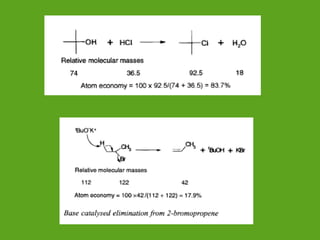
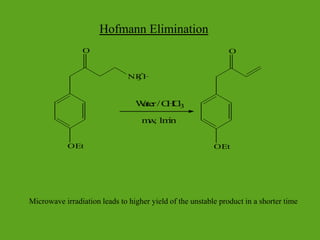
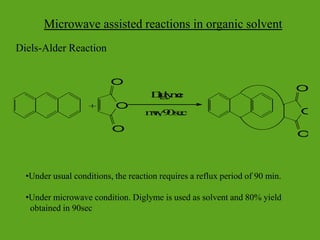
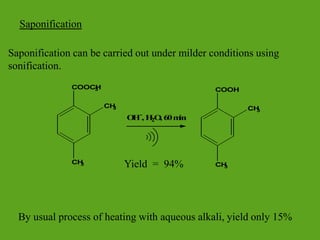
La química verde se centra en la reducción o eliminación de sustancias peligrosas en el diseño, fabricación y aplicación de productos químicos, buscando prevenir la contaminación desde su origen. Incluye doce principios, como la minimización de residuos, el uso de materiales más seguros y la eficiencia energética, y propone métodos de síntesis más sostenibles. Aunque no resuelve todos los problemas ambientales, la química verde es un enfoque fundamental para la prevención de la contaminación.