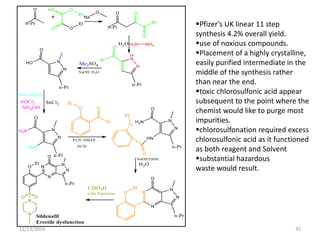
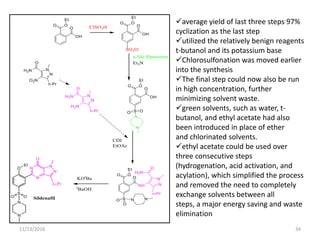
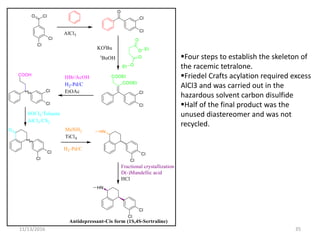
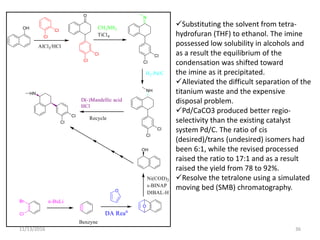
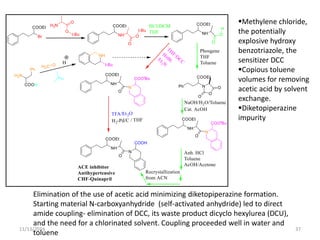
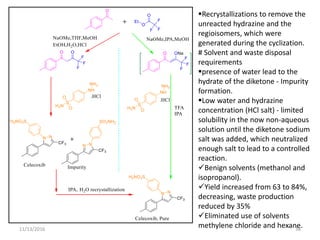
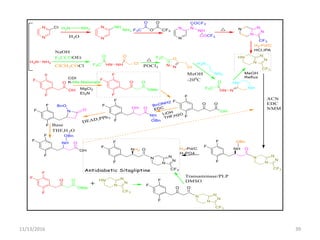
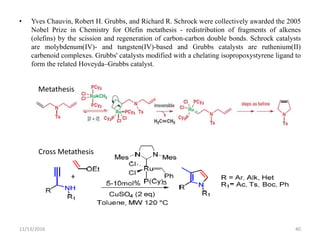
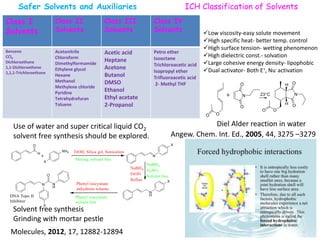
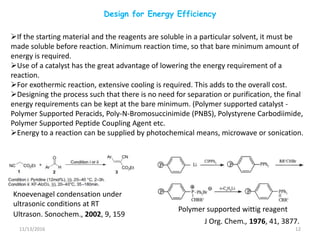
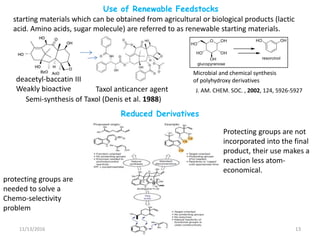
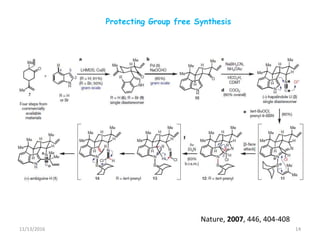
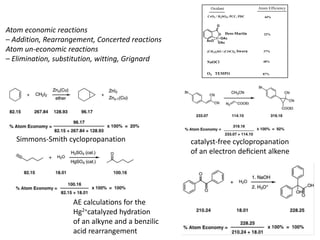
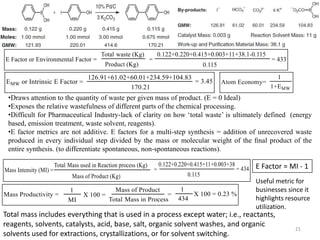
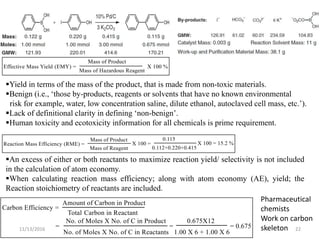
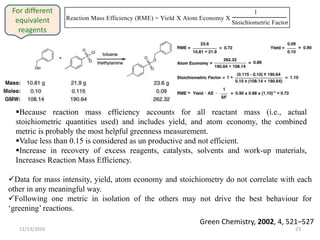
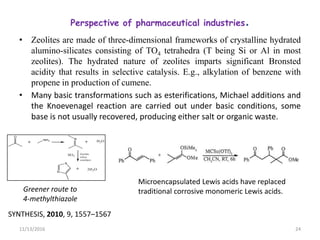
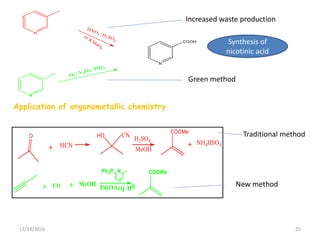
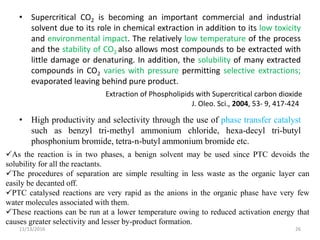
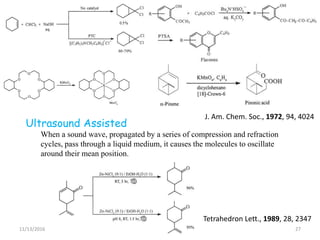
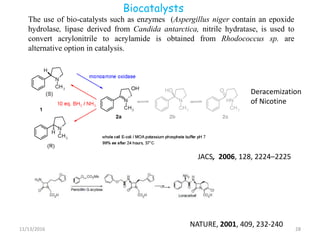
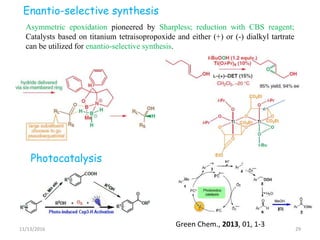
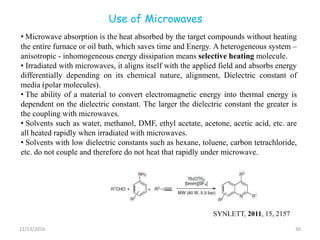
The document discusses green chemistry, outlining its principles, metrics, and implications for the pharmaceutical industry. It highlights historical chemical hazards, such as DDT and thalidomide, and advocates for safer, more environmentally friendly chemical processes. The adoption of green chemistry aims to minimize waste, use renewable materials, and reduce the toxicity of chemicals, while also ensuring efficient production methods.








![Ionic Liquids
11/13/2016 31
Quaternization of an Amine or Phosphane followed by Lewis Acid
Multiphasic system, different selectivities, easy separation.
Heck Reaction- Synlett., 1996, 11, 1091
Beckmann Rearrangement-
Tetrahedron Letters, 2004, 45, 2681
Baeyer villiger rearrangement
Tetra Letters, 2003, 44, 8991
e. g., [emin]CI-AICI3 , their m.p.
and properties depend upon the
mole fractions of AICl3
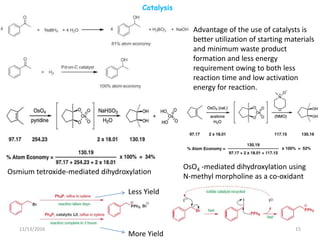
and 1 ,3-dialkyl imidazolium
chloride present.](https://image.slidesharecdn.com/greenchemmcphd201608-161113150901/85/Green-chemistry-31-320.jpg)