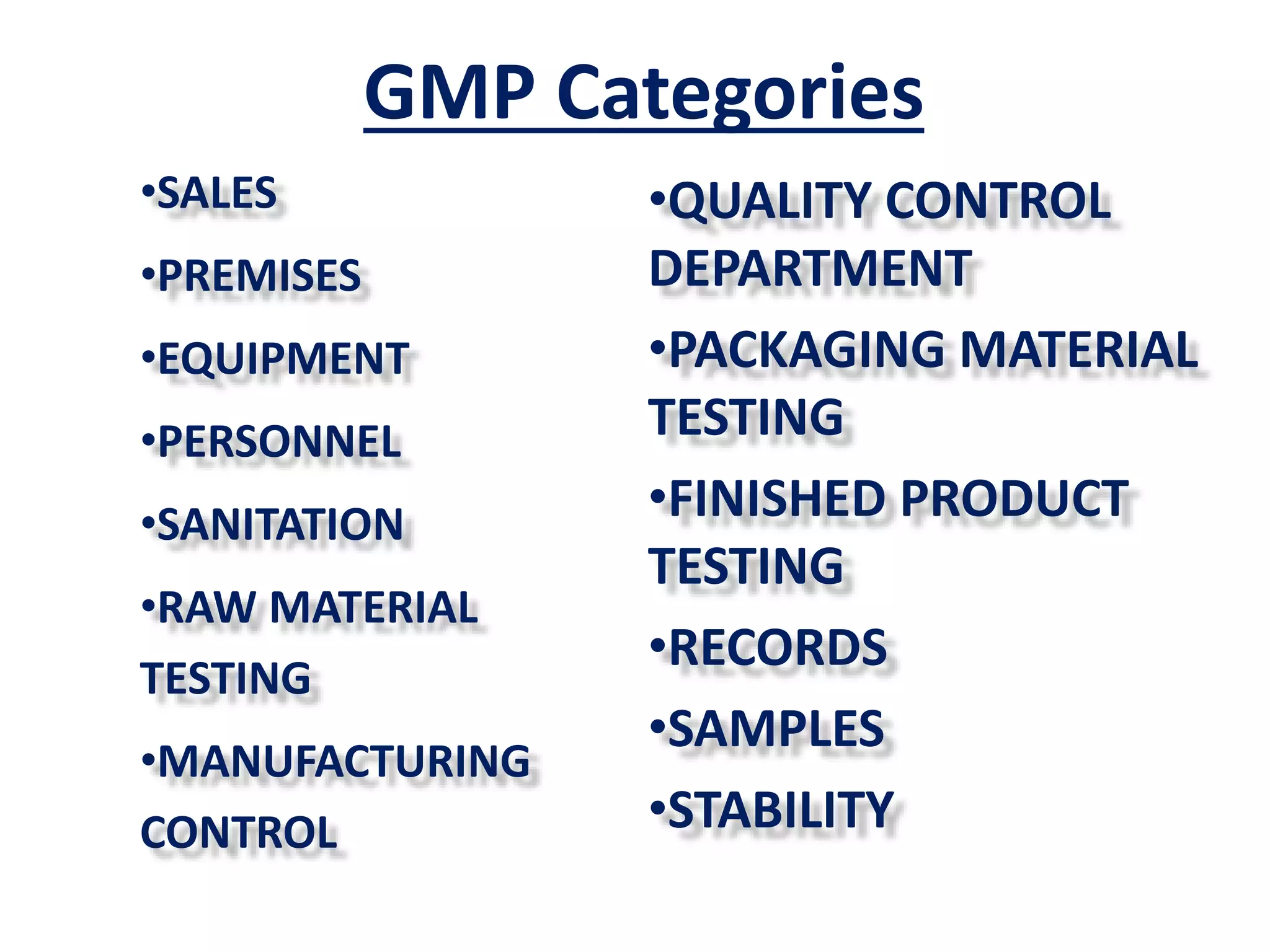
This document provides an overview of the history and development of Good Manufacturing Practices (GMP) regulations. It discusses key events that shaped GMP standards, including unsanitary meat packing conditions exposed in 1905, the sulfathiazole tablet contamination incident in 1941 that resulted in the formal adoption of GMP, and other drug and medical device issues in subsequent decades. The document also outlines GMP requirements regarding facilities, equipment, personnel, sanitation, materials testing, manufacturing controls, quality control, records, and other areas. Overall, it traces how GMP evolved from early 1900s to modern standards to ensure consistency and quality in pharmaceutical manufacturing.