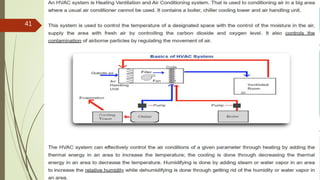
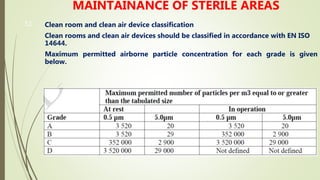
The document outlines GMP requirements related to personnel, training, hygiene, premises design and layout, warehouse, production and auxiliary areas, and quality control. It emphasizes that facilities, equipment, and personnel must be suitable for manufacturing pharmaceutical products and maintaining quality. Key responsibilities are defined for heads of production and quality units to ensure compliance with GMP standards.