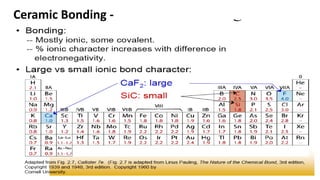
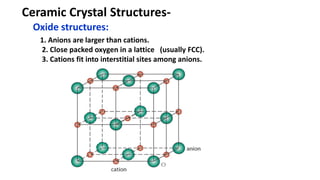
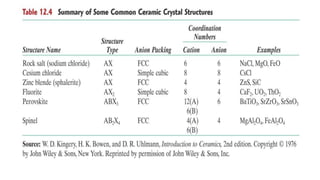
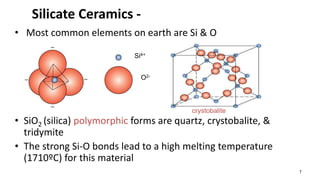
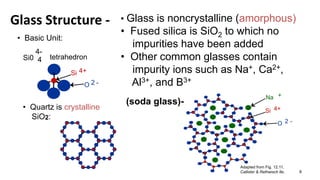
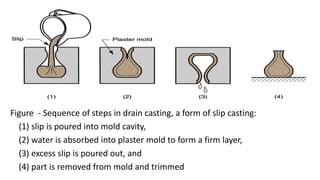
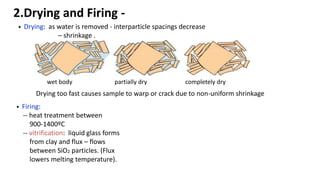
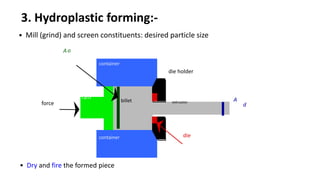
The document discusses ceramics as inorganic compounds made from metal and nonmetal elements, highlighting their properties, processing methods, and various applications. Key ceramic materials include silica, alumina, and their complex compounds, with methods for forming such as slip casting and powder pressing. Applications range from construction materials to electrical insulators and cutting tools, showcasing their versatility in high-temperature and structural applications.